Electrons and Quantum Mechanics - Oakland Schools
Electrons and Quantum Mechanics - Oakland Schools
Electrons and Quantum Mechanics - Oakland Schools
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Disposal<br />
Discussion<br />
Solutions may be flushed down the drain with ample water.<br />
In water solutions, transition metal ions are usually not found as single ions but<br />
as complex ions in which the metal ion is bonded to 2, 4, or 6 water molecules<br />
with coordinate covalent bonds. If the water solution is mixed with another<br />
species which can donate electrons to the metal, a new complex ion with the<br />
new species may be formed. The various species which can complex with metal<br />
ions are referred to as lig<strong>and</strong>s. Often the color of the new complex ion is<br />
different from the original color of the metal ion in water.<br />
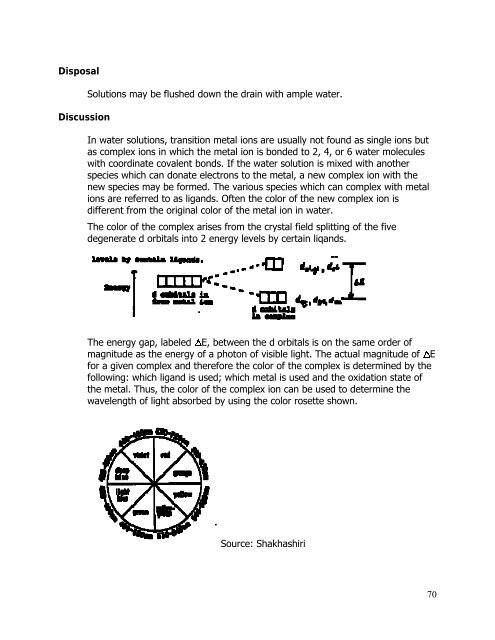
The color of the complex arises from the crystal field splitting of the five<br />
degenerate d orbitals into 2 energy levels by certain liq<strong>and</strong>s.<br />
The energy gap, labeled E, between the d orbitals is on the same order of<br />
magnitude as the energy of a photon of visible light. The actual magnitude of E<br />
for a given complex <strong>and</strong> therefore the color of the complex is determined by the<br />
following: which lig<strong>and</strong> is used; which metal is used <strong>and</strong> the oxidation state of<br />
the metal. Thus, the color of the complex ion can be used to determine the<br />
wavelength of light absorbed by using the color rosette shown.<br />
Source: Shakhashiri<br />
70