Electrons and Quantum Mechanics - Oakland Schools
Electrons and Quantum Mechanics - Oakland Schools
Electrons and Quantum Mechanics - Oakland Schools
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
“Und” is the undiscovered inert element that would be below radon on the periodic<br />
chart.<br />
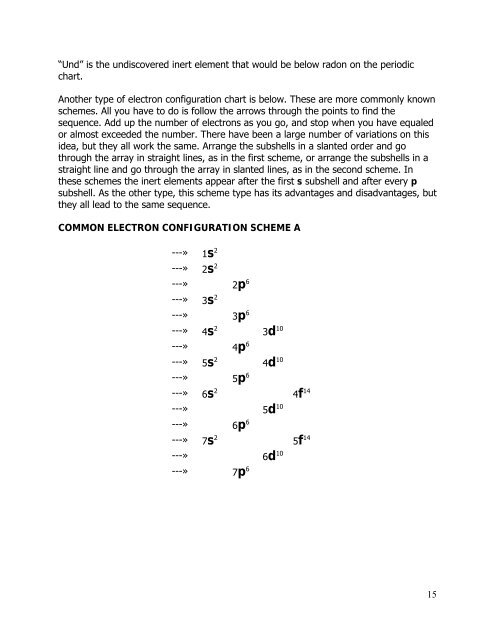
Another type of electron configuration chart is below. These are more commonly known<br />
schemes. All you have to do is follow the arrows through the points to find the<br />
sequence. Add up the number of electrons as you go, <strong>and</strong> stop when you have equaled<br />
or almost exceeded the number. There have been a large number of variations on this<br />
idea, but they all work the same. Arrange the subshells in a slanted order <strong>and</strong> go<br />
through the array in straight lines, as in the first scheme, or arrange the subshells in a<br />
straight line <strong>and</strong> go through the array in slanted lines, as in the second scheme. In<br />
these schemes the inert elements appear after the first s subshell <strong>and</strong> after every p<br />
subshell. As the other type, this scheme type has its advantages <strong>and</strong> disadvantages, but<br />
they all lead to the same sequence.<br />
COMMON ELECTRON CONFIGURATION SCHEME A<br />
---» 1s 2<br />
---» 2s 2<br />
---» 2p 6<br />
---» 3s 2<br />
---» 3p 6<br />
---» 4s 2 3d 10<br />
---» 4p 6<br />
---» 5s 2 4d 10<br />
---» 5p 6<br />
---» 6s 2 4f 14<br />
---» 5d 10<br />
---» 6p 6<br />
---» 7s 2 5f 14<br />
---» 6d 10<br />
---» 7p 6<br />
15