Electrons and Quantum Mechanics - Oakland Schools
Electrons and Quantum Mechanics - Oakland Schools
Electrons and Quantum Mechanics - Oakland Schools
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
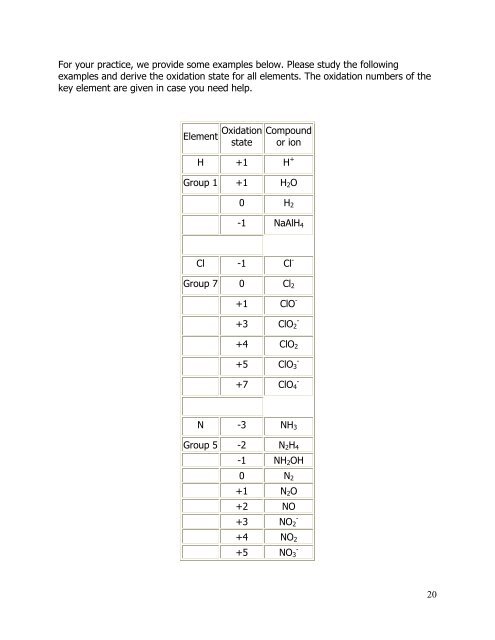
For your practice, we provide some examples below. Please study the following<br />
examples <strong>and</strong> derive the oxidation state for all elements. The oxidation numbers of the<br />
key element are given in case you need help.<br />
Element Oxidation<br />
state<br />
Compound<br />
or ion<br />
H +1 H +<br />
Group 1 +1 H 2 O<br />
0 H 2<br />
-1 NaAlH 4<br />
Cl -1 Cl -<br />
Group 7 0 Cl 2<br />
+1 ClO -<br />
+3 ClO 2<br />
-<br />
+4 ClO 2<br />
+5 ClO 3<br />
-<br />
+7 ClO 4<br />
-<br />
N -3 NH 3<br />
Group 5 -2 N 2 H 4<br />
-1 NH 2 OH<br />
0 N 2<br />
+1 N 2 O<br />
+2 NO<br />
+3<br />
-<br />
NO 2<br />
+4 NO 2<br />
+5<br />
-<br />
NO 3<br />
20