Electrons and Quantum Mechanics - Oakland Schools
Electrons and Quantum Mechanics - Oakland Schools
Electrons and Quantum Mechanics - Oakland Schools
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Discussion<br />
Chemiluminescence is defined as the production or emission of light that accompanies a<br />
chemical reaction. Light emission results from the conversion of chemical energy into<br />
light energy due to changes in the composition of a chemiluminescent material.<br />
The “flame test” colors observed when different metal salts are burned in a Bunsen<br />
burner flame are examples of a type of chemiluminescence known as<br />
pyroluminescence. The oxidation of luminol (3-aminophthalhydrazide) in this<br />
demonstration is another well-known example of chemiluminescence. The lightproducing<br />
chemical reactions of luminol were discovered by H. O. Albrecht in 1928.<br />
Since that time numerous procedures have been developed to produce light using<br />
luminol. Experiments have shown that the following “ingredients” are necessary for<br />
luminol to exhibit chemiluminescence—a basic (alkaline) pH, an oxidizing agent, <strong>and</strong> a<br />
catalyst. In this demonstration, the oxidizing agent is Clorox 2, which also maintains the<br />
basic pH needed, <strong>and</strong> the catalyst is the iron(III) cation in potassium ferricyanide.<br />
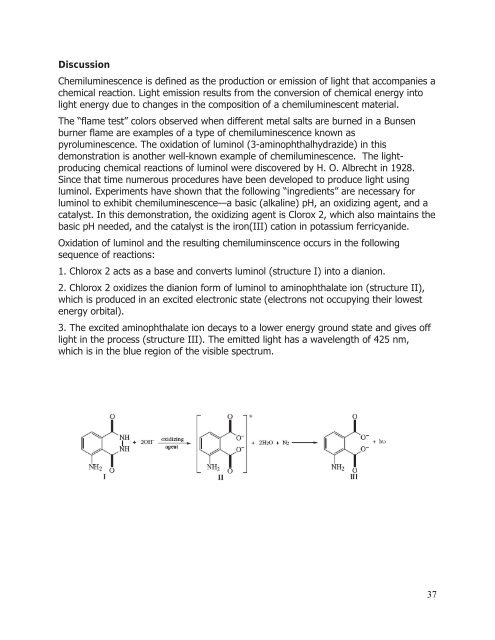
Oxidation of luminol <strong>and</strong> the resulting chemiluminscence occurs in the following<br />
sequence of reactions:<br />
1. Chlorox 2 acts as a base <strong>and</strong> converts luminol (structure I) into a dianion.<br />
2. Chlorox 2 oxidizes the dianion form of luminol to aminophthalate ion (structure II),<br />
which is produced in an excited electronic state (electrons not occupying their lowest<br />
energy orbital).<br />
3. The excited aminophthalate ion decays to a lower energy ground state <strong>and</strong> gives off<br />
light in the process (structure III). The emitted light has a wavelength of 425 nm,<br />
which is in the blue region of the visible spectrum.<br />
37