Electrons and Quantum Mechanics - Oakland Schools
Electrons and Quantum Mechanics - Oakland Schools
Electrons and Quantum Mechanics - Oakland Schools
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Materials<br />
(For 24 students working in pairs)<br />
24 Targets (see sample targets in Appendix)<br />
12 Typewriter carbon papers<br />
48 Paper clips<br />
12 Marbles<br />
Safety Concerns<br />
There are no unusual safety requirements, except to insure that students with marbledrop<br />
responsibilities climb carefully onto stable, safe structures.<br />
Source<br />
http://dwb4.unl.edu/chem_source_pdf/ChemSource.html<br />
Procedure<br />
Data Analysis <strong>and</strong> Concept Development<br />
1. Locate the 95 dots (95% of the 100) that are closest to the bulls-eye. Using a pencil,<br />
draw a smooth (not wavy) curve to enclose the region containing these dots. This<br />
region represents the two-dimensional “orbital” of your “marble electron” (analogous to<br />
the region of space where an electron might be observed 95% of the time in an<br />
experiment to locate it in three-dimensional space).<br />
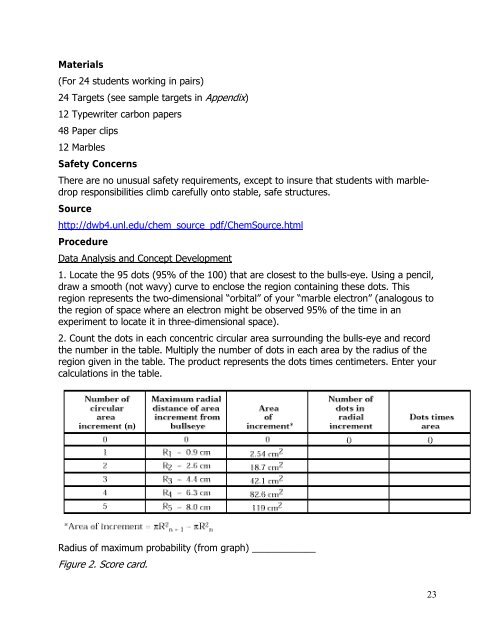
2. Count the dots in each concentric circular area surrounding the bulls-eye <strong>and</strong> record<br />
the number in the table. Multiply the number of dots in each area by the radius of the<br />
region given in the table. The product represents the dots times centimeters. Enter your<br />
calculations in the table.<br />
Radius of maximum probability (from graph) ____________<br />
Figure 2. Score card.<br />
23