The Czech Republic Annual Report 2010 Drug ... - Drogy-info.cz
The Czech Republic Annual Report 2010 Drug ... - Drogy-info.cz
The Czech Republic Annual Report 2010 Drug ... - Drogy-info.cz
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
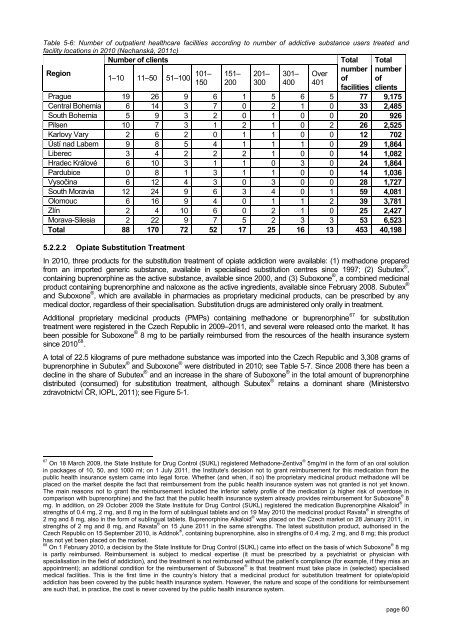
Table 5-6: Number of outpatient healthcare facilities according to number of addictive substance users treated and<br />
facility locations in <strong>2010</strong> (Nechanská, 2011c)<br />
Number of clients<br />
Region<br />
1–10 11–50 51–100 101–<br />
150<br />
151–<br />
200<br />
201–<br />
300<br />
301–<br />
400<br />
Over<br />
401<br />
Total<br />
number<br />
of<br />
facilities<br />
Total<br />
number<br />
of<br />
clients<br />
Prague 19 26 9 6 1 5 6 5 77 9,175<br />
Central Bohemia 6 14 3 7 0 2 1 0 33 2,485<br />
South Bohemia 5 9 3 2 0 1 0 0 20 926<br />
Pilsen 10 7 3 1 2 1 0 2 26 2,525<br />
Karlovy Vary 2 6 2 0 1 1 0 0 12 702<br />
Ústí nad Labem 9 8 5 4 1 1 1 0 29 1,864<br />
Liberec 3 4 2 2 2 1 0 0 14 1,082<br />
Hradec Králové 6 10 3 1 1 0 3 0 24 1,864<br />
Pardubice 0 8 1 3 1 1 0 0 14 1,036<br />
Vysočina 6 12 4 3 0 3 0 0 28 1,727<br />
South Moravia 12 24 9 6 3 4 0 1 59 4,081<br />
Olomouc 6 16 9 4 0 1 1 2 39 3,781<br />
Zlín 2 4 10 6 0 2 1 0 25 2,427<br />
Morava-Silesia 2 22 9 7 5 2 3 3 53 6,523<br />
Total 88 170 72 52 17 25 16 13 453 40,198<br />
5.2.2.2 Opiate Substitution Treatment<br />
In <strong>2010</strong>, three products for the substitution treatment of opiate addiction were available: (1) methadone prepared<br />
from an imported generic substance, available in specialised substitution centres since 1997; (2) Subutex ® ,<br />
containing buprenorphine as the active substance, available since 2000, and (3) Suboxone ® , a combined medicinal<br />
product containing buprenorphine and naloxone as the active ingredients, available since February 2008. Subutex ®<br />
and Suboxone ® , which are available in pharmacies as proprietary medicinal products, can be prescribed by any<br />
medical doctor, regardless of their specialisation. Substitution drugs are administered only orally in treatment.<br />
Additional proprietary medicinal products (PMPs) containing methadone or buprenorphine 67 for substitution<br />
treatment were registered in the <strong>Czech</strong> <strong>Republic</strong> in 2009–2011, and several were released onto the market. It has<br />
been possible for Suboxone ® 8 mg to be partially reimbursed from the resources of the health insurance system<br />
since <strong>2010</strong> 68 .<br />
A total of 22.5 kilograms of pure methadone substance was imported into the <strong>Czech</strong> <strong>Republic</strong> and 3,308 grams of<br />
buprenorphine in Subutex ® and Suboxone ® were distributed in <strong>2010</strong>; see Table 5-7. Since 2008 there has been a<br />
decline in the share of Subutex ® and an increase in the share of Suboxone ® in the total amount of buprenorphine<br />
distributed (consumed) for substitution treatment, although Subutex ® retains a dominant share (Ministerstvo<br />
zdravotnictví ČR, IOPL, 2011); see Figure 5-1.<br />
67<br />
On 18 March 2009, the State Institute for <strong>Drug</strong> Control (SUKL) registered Methadone-Zentiva ® 5mg/ml in the form of an oral solution<br />
in packages of 10, 50, and 1000 ml; on 1 July 2011, the Institute's decision not to grant reimbursement for this medication from the<br />
public health insurance system came into legal force. Whether (and when, if so) the proprietary medicinal product methadone will be<br />
placed on the market despite the fact that reimbursement from the public health insurance system was not granted is not yet known.<br />
<strong>The</strong> main reasons not to grant the reimbursement included the inferior safety profile of the medication (a higher risk of overdose in<br />
comparison with buprenorphine) and the fact that the public health insurance system already provides reimbursement for Suboxone ® 8<br />
mg. In addition, on 29 October 2009 the State Institute for <strong>Drug</strong> Control (SUKL) registered the medication Buprenorphine Alkaloid ® in<br />
strengths of 0.4 mg, 2 mg, and 8 mg in the form of sublingual tablets and on 19 May <strong>2010</strong> the medicinal product Ravata ® in strengths of<br />
2 mg and 8 mg, also in the form of sublingual tablets. Buprenorphine Alkaloid ® was placed on the <strong>Czech</strong> market on 28 January 2011, in<br />
strengths of 2 mg and 8 mg, and Ravata ® on 15 June 2011 in the same strengths. <strong>The</strong> latest substitution product, authorised in the<br />
<strong>Czech</strong> <strong>Republic</strong> on 15 September <strong>2010</strong>, is Addnok ® , containing buprenorphine, also in strengths of 0.4 mg, 2 mg, and 8 mg; this product<br />
has not yet been placed on the market.<br />
68<br />
On 1 February <strong>2010</strong>, a decision by the State Institute for <strong>Drug</strong> Control (SUKL) came into effect on the basis of which Suboxone ® 8 mg<br />
is partly reimbursed. Reimbursement is subject to medical expertise (it must be prescribed by a psychiatrist or physician with<br />
specialisation in the field of addiction), and the treatment is not reimbursed without the patient’s compliance (for example, if they miss an<br />
appointment); an additional condition for the reimbursement of Suboxone ® is that treatment must take place in (selected) specialised<br />
medical facilities. This is the first time in the country’s history that a medicinal product for substitution treatment for opiate/opioid<br />
addiction has been covered by the public health insurance system. However, the nature and scope of the conditions for reimbursement<br />
are such that, in practice, the cost is never covered by the public health insurance system.<br />
page 60