DID 60 standard roller chain - Big Bike Webshop
DID 60 standard roller chain - Big Bike Webshop
DID 60 standard roller chain - Big Bike Webshop
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
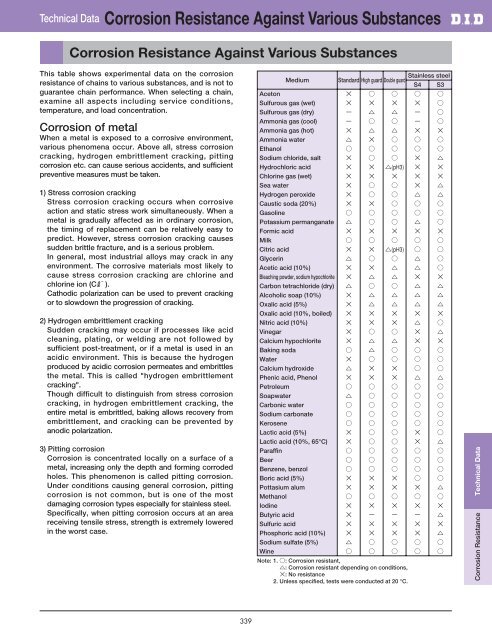
Technical Data Corrosion Resistance Against Various Substances<br />
Corrosion Resistance Against Various Substances<br />
This table shows experimental data on the corrosion<br />
resistance of <strong>chain</strong>s to various substances, and is not to<br />
guarantee <strong>chain</strong> performance. When selecting a <strong>chain</strong>,<br />
examine all aspects including service conditions,<br />
temperature, and load concentration.<br />
Corrosion of metal<br />
When a metal is exposed to a corrosive environment,<br />
various phenomena occur. Above all, stress corrosion<br />
cracking, hydrogen embrittlement cracking, pitting<br />
corrosion etc. can cause serious accidents, and sufficient<br />
preventive measures must be taken.<br />
1) Stress corrosion cracking<br />
Stress corrosion cracking occurs when corrosive<br />
action and static stress work simultaneously. When a<br />
metal is gradually affected as in ordinary corrosion,<br />
the timing of replacement can be relatively easy to<br />
predict. However, stress corrosion cracking causes<br />
sudden brittle fracture, and is a serious problem.<br />
In general, most industrial alloys may crack in any<br />
environment. The corrosive materials most likely to<br />
cause stress corrosion cracking are chlorine and<br />
chlorine ion (CR – ).<br />
Cathodic polarization can be used to prevent cracking<br />
or to slowdown the progression of cracking.<br />
2) Hydrogen embrittlement cracking<br />
Sudden cracking may occur if processes like acid<br />
cleaning, plating, or welding are not followed by<br />
sufficient post-treatment, or if a metal is used in an<br />
acidic environment. This is because the hydrogen<br />
produced by acidic corrosion permeates and embrittles<br />
the metal. This is called "hydrogen embrittlement<br />
cracking".<br />
Though difficult to distinguish from stress corrosion<br />
cracking, in hydrogen embrittlement cracking, the<br />
entire metal is embrittled, baking allows recovery from<br />
embrittlement, and cracking can be prevented by<br />
anodic polarization.<br />
3) Pitting corrosion<br />
Corrosion is concentrated locally on a surface of a<br />
metal, increasing only the depth and forming corroded<br />
holes. This phenomenon is called pitting corrosion.<br />
Under conditions causing general corrosion, pitting<br />
corrosion is not common, but is one of the most<br />
damaging corrosion types especially for stainless steel.<br />
Specifically, when pitting corrosion occurs at an area<br />
receiving tensile stress, strength is extremely lowered<br />
in the worst case.<br />
Medium<br />
Aceton<br />
Sulfurous gas (wet)<br />
Sulfurous gas (dry)<br />
Ammonia gas (cool)<br />
Ammonia gas (hot)<br />
Ammonia water<br />
Ethanol<br />
Sodium chloride, salt<br />
Hydrochloric acid<br />
Chlorine gas (wet)<br />
Sea water<br />
Hydrogen peroxide<br />
Caustic soda (20%)<br />
Gasoline<br />
Potassium permanganate<br />
Formic acid<br />
Milk<br />
Citric acid<br />
Glycerin<br />
Acetic acid (10%)<br />
Bleaching powder, sodium hypochlorite<br />
Carbon tetrachloride (dry)<br />
Alcoholic soap (10%)<br />
Oxalic acid (5%)<br />
Oxalic acid (10%, boiled)<br />
Nitric acid (10%)<br />
Vinegar<br />
Calcium hypochlorite<br />
Baking soda<br />
Water<br />
Calcium hydroxide<br />
Phenic acid, Phenol<br />
Petroleum<br />
Soapwater<br />
Carbonic water<br />
Sodium carbonate<br />
Kerosene<br />
Lactic acid (5%)<br />
Lactic acid (10%, 65°C)<br />
Paraffin<br />
Beer<br />
Benzene, benzol<br />
Boric acid (5%)<br />
Pottasium alum<br />
Methanol<br />
Iodine<br />
Butyric acid<br />
Sulfuric acid<br />
Phosphoric acid (10%)<br />
Sodium sulfate (5%)<br />
Wine<br />
Stainless steel<br />
Standard High guard Double guard<br />
S3<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
Note: 1. : Corrosion resistant,<br />
: Corrosion resistant depending on conditions,<br />
: No resistance<br />
2. Unless specified, tests were conducted at 20 °C.<br />
S4<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
Technical Data<br />
Corrosion Resistance<br />
339