Cockroache; Ecology, behavior & history - W.J. Bell
Cockroache; Ecology, behavior & history - W.J. Bell
Cockroache; Ecology, behavior & history - W.J. Bell
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
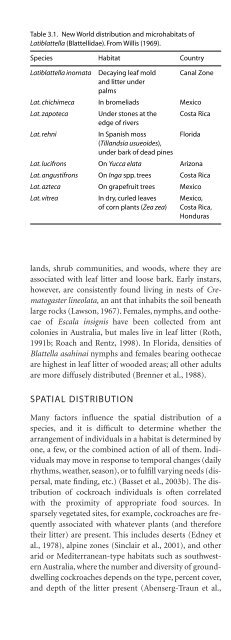
Table 3.1. New World distribution and microhabitats of<br />
Latiblattella (Blattellidae). From Willis (1969).<br />
Species Habitat Country<br />
Latiblattella inornata Decaying leaf mold Canal Zone<br />
and litter under<br />
palms<br />
Lat. chichimeca In bromeliads Mexico<br />
Lat. zapoteca Under stones at the Costa Rica<br />
edge of rivers<br />
Lat. rehni In Spanish moss Florida<br />
(Tillandsia usueoides),<br />
under bark of dead pines<br />
Lat. lucifrons On Yucca elata Arizona<br />
Lat. angustifrons On Inga spp. trees Costa Rica<br />
Lat. azteca On grapefruit trees Mexico<br />
Lat. vitrea In dry, curled leaves Mexico,<br />
of corn plants (Zea zea) Costa Rica,<br />
Honduras<br />
1996a). In wood-feeding cockroaches, juvenile food and<br />
habitat is set when the parent chooses a log to colonize.<br />
The horizontal distribution of cockroaches in caves is often<br />
related to the resting positions of bats, which determine<br />
the placement of guano and other organic matter.<br />
Gautier (1974a, 1974b) calculated the spatial distribution<br />
of burrowing Blaberus nymphs in caves by counting the<br />
number of individuals in 50 cm 2 samples to a depth of 15<br />
cm. He found that nymphs were concentrated in zones<br />
where bat guano, fruit, and twigs dropped by the bats<br />
accumulated, and were absent from zones of dry soil,<br />
stones, or pebbles. In many cave cockroaches, females descend<br />
from their normal perches on the cave walls to<br />
oviposit or give birth on the cave floor in or near guano<br />
(e.g., Blaberus, Eublaberus, Periplaneta—Crawford and<br />
Cloudsley-Thompson, 1971; Gautier, 1974b; Deleporte,<br />
1976), where the nymphs remain until they are at least<br />
half grown. They then climb onto the cave walls, where<br />
they complete their development.<br />
CIRCADIAN ACTIVITY<br />
lands, shrub communities, and woods, where they are<br />
associated with leaf litter and loose bark. Early instars,<br />
however, are consistently found living in nests of Crematogaster<br />
lineolata, an ant that inhabits the soil beneath<br />
large rocks (Lawson, 1967). Females, nymphs, and oothecae<br />
of Escala insignis have been collected from ant<br />
colonies in Australia, but males live in leaf litter (Roth,<br />
1991b; Roach and Rentz, 1998). In Florida, densities of<br />
Blattella asahinai nymphs and females bearing oothecae<br />
are highest in leaf litter of wooded areas; all other adults<br />
are more diffusely distributed (Brenner et al., 1988).<br />
SPATIAL DISTRIBUTION<br />
Many factors influence the spatial distribution of a<br />
species, and it is difficult to determine whether the<br />
arrangement of individuals in a habitat is determined by<br />
one, a few, or the combined action of all of them. Individuals<br />
may move in response to temporal changes (daily<br />
rhythms, weather, season), or to fulfill varying needs (dispersal,<br />
mate finding, etc.) (Basset et al., 2003b). The distribution<br />
of cockroach individuals is often correlated<br />
with the proximity of appropriate food sources. In<br />
sparsely vegetated sites, for example, cockroaches are frequently<br />
associated with whatever plants (and therefore<br />
their litter) are present. This includes deserts (Edney et<br />
al., 1978), alpine zones (Sinclair et al., 2001), and other<br />
arid or Mediterranean-type habitats such as southwestern<br />
Australia, where the number and diversity of grounddwelling<br />
cockroaches depends on the type, percent cover,<br />
and depth of the litter present (Abenserg-Traun et al.,<br />
Many species exhibit daily and seasonal movements in response<br />
to their dietary, reproductive, and microenvironmental<br />
needs; these vary with the individual, sex, developmental<br />
stage, species, day, season, and habitat. Activity<br />
patterns are expected to differ, for instance, between those<br />
cockroaches that forage, find mates, reproduce, and take<br />
refuge all in the same habitat (in logs, under bark, in leaf<br />
litter) and those that move daily between their harborage<br />
and the habitats in which they conduct most other life activities.<br />
The most common circadian activity pattern<br />
among the latter is for nymphs and adults to rest in<br />
harborages during the day, then become active as the sun<br />
sets. At dusk, adults climb or fly to above-ground perching<br />
sites (Schal and <strong>Bell</strong>, 1986), while nymphs confine<br />
their activities to the leaf litter. Some species are evidently<br />
active for short periods just after sunset, whereas others<br />
may be observed throughout the night. Within 60 min after<br />
sunset, adult males and small nymphs of Periplaneta<br />
fuliginosa emerge from their harborage, followed by<br />
medium and large nymphs and adult females. After feeding,<br />
males climb vertical surfaces, while nymphs and most<br />
females return to shelter (Appel and Rust, 1986). Males<br />
also become active earlier than females in Ectobius lapponicus.<br />
They begin moving in the late afternoon, while<br />
females and nymphs wait until after sunset (Dreisig,<br />
1971). In Nesomylacris sp., most females do not become<br />
active until just before dawn, while males are active<br />
throughout the night. Females of Epilampra involucris are<br />
active at both dusk and dawn (Fig. 3.2). With few exceptions,<br />
temporal overlap among nocturnally active species<br />
is large.<br />
HABITATS 39