FilmTec Technical Manual - Chester Paul Company
FilmTec Technical Manual - Chester Paul Company
FilmTec Technical Manual - Chester Paul Company
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
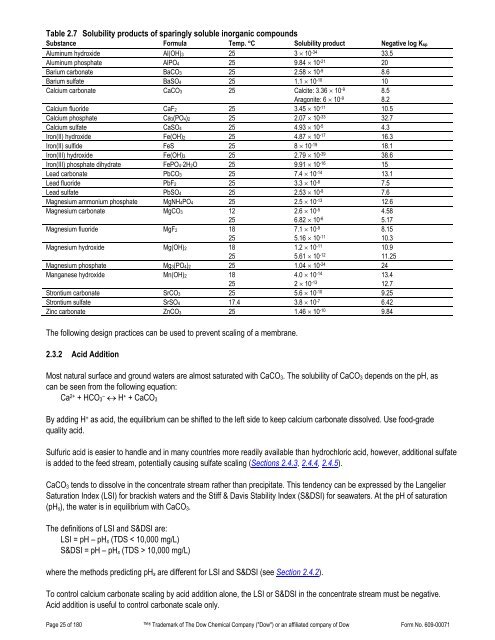
Table 2.7 Solubility products of sparingly soluble inorganic compoundsSubstance Formula Temp. °C Solubility product Negative log KspAluminum hydroxide Al(OH)3 25 3 × 10 -34 33.5Aluminum phosphate AlPO4 25 9.84 × 10 -21 20Barium carbonate BaCO3 25 2.58 × 10 -9 8.6Barium sulfate BaSO4 25 1.1 × 10 -10 10Calcium carbonate CaCO3 25 Calcite: 3.36 × 10 -9Aragonite: 6 × 10 -98.58.2Calcium fluoride CaF2 25 3.45 × 10 -11 10.5Calcium phosphate Ca3(PO4)2 25 2.07 × 10 -33 32.7Calcium sulfate CaSO4 25 4.93 × 10 -5 4.3Iron(II) hydroxide Fe(OH)2 25 4.87 × 10 -17 16.3Iron(II) sulfide FeS 25 8 × 10 -19 18.1Iron(III) hydroxide Fe(OH)3 25 2.79 × 10 -39 38.6Iron(III) phosphate dihydrate FePO4⋅2H2O 25 9.91 × 10 -16 15Lead carbonate PbCO3 25 7.4 × 10 -14 13.1Lead fluoride PbF2 25 3.3 × 10 -8 7.5Lead sulfate PbSO4 25 2.53 × 10 -8 7.6Magnesium ammonium phosphate MgNH4PO4 25 2.5 × 10 -13 12.6Magnesium carbonate MgCO3 12252.6 × 10 -56.82 × 10 -64.585.17Magnesium fluoride MgF2 18257.1 × 10 -95.16 × 10 -118.1510.3Magnesium hydroxide Mg(OH)2 18251.2 × 10 -115.61 × 10 -1210.911.25Magnesium phosphate Mg3(PO4)2 25 1.04 × 10 -24 24Manganese hydroxide Mn(OH)2 18254.0 × 10 -142 × 10 -1313.412.7Strontium carbonate SrCO3 25 5.6 × 10 -10 9.25Strontium sulfate SrSO4 17.4 3.8 × 10 -7 6.42Zinc carbonate ZnCO3 25 1.46 × 10 -10 9.84The following design practices can be used to prevent scaling of a membrane.2.3.2 Acid AdditionMost natural surface and ground waters are almost saturated with CaCO 3 . The solubility of CaCO 3 depends on the pH, ascan be seen from the following equation:Ca 2+ + HCO 3–↔ H + + CaCO 3By adding H + as acid, the equilibrium can be shifted to the left side to keep calcium carbonate dissolved. Use food-gradequality acid.Sulfuric acid is easier to handle and in many countries more readily available than hydrochloric acid, however, additional sulfateis added to the feed stream, potentially causing sulfate scaling (Sections 2.4.3, 2.4.4, 2.4.5).CaCO 3 tends to dissolve in the concentrate stream rather than precipitate. This tendency can be expressed by the LangelierSaturation Index (LSI) for brackish waters and the Stiff & Davis Stability Index (S&DSI) for seawaters. At the pH of saturation(pH s ), the water is in equilibrium with CaCO 3 .The definitions of LSI and S&DSI are:LSI = pH – pH s (TDS < 10,000 mg/L)S&DSI = pH – pH s (TDS > 10,000 mg/L)where the methods predicting pH s are different for LSI and S&DSI (see Section 2.4.2).To control calcium carbonate scaling by acid addition alone, the LSI or S&DSI in the concentrate stream must be negative.Acid addition is useful to control carbonate scale only.Page 25 of 180 ® Trademark of The Dow Chemical <strong>Company</strong> ("Dow") or an affiliated company of Dow Form No. 609-00071