Uranium ore-forming systems of the - Geoscience Australia
Uranium ore-forming systems of the - Geoscience Australia
Uranium ore-forming systems of the - Geoscience Australia
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
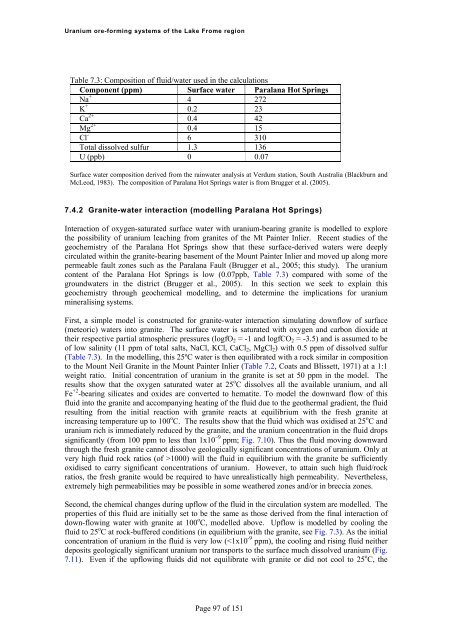
<strong>Uranium</strong> <strong>ore</strong>-<strong>forming</strong> <strong>systems</strong> <strong>of</strong> <strong>the</strong> Lake Frome regionTable 7.3: Composition <strong>of</strong> fluid/water used in <strong>the</strong> calculationsComponent (ppm) Surface water Paralana Hot SpringsNa + 4 272K + 0.2 23Ca 2+ 0.4 42Mg 2+ 0.4 15Cl - 6 310Total dissolved sulfur 1.3 136U (ppb) 0 0.07Surface water composition derived from <strong>the</strong> rainwater analysis at Verdum station, South <strong>Australia</strong> (Blackburn andMcLeod, 1983). The composition <strong>of</strong> Paralana Hot Springs water is from Brugger et al. (2005).7.4.2 Granite-water interaction (modelling Paralana Hot Springs)Interaction <strong>of</strong> oxygen-saturated surface water with uranium-bearing granite is modelled to expl<strong>ore</strong><strong>the</strong> possibility <strong>of</strong> uranium leaching from granites <strong>of</strong> <strong>the</strong> Mt Painter Inlier. Recent studies <strong>of</strong> <strong>the</strong>geochemistry <strong>of</strong> <strong>the</strong> Paralana Hot Springs show that <strong>the</strong>se surface-derived waters were deeplycirculated within <strong>the</strong> granite-bearing basement <strong>of</strong> <strong>the</strong> Mount Painter Inlier and moved up along m<strong>ore</strong>permeable fault zones such as <strong>the</strong> Paralana Fault (Brugger et al., 2005; this study). The uraniumcontent <strong>of</strong> <strong>the</strong> Paralana Hot Springs is low (0.07ppb, Table 7.3) compared with some <strong>of</strong> <strong>the</strong>groundwaters in <strong>the</strong> district (Brugger et al., 2005). In this section we seek to explain thisgeochemistry through geochemical modelling, and to determine <strong>the</strong> implications for uraniummineralising <strong>systems</strong>.First, a simple model is constructed for granite-water interaction simulating downflow <strong>of</strong> surface(meteoric) waters into granite. The surface water is saturated with oxygen and carbon dioxide at<strong>the</strong>ir respective partial atmospheric pressures (logfO 2 = -1 and logfCO 2 = -3.5) and is assumed to be<strong>of</strong> low salinity (11 ppm <strong>of</strong> total salts, NaCl, KCl, CaCl 2 , MgCl 2 ) with 0.5 ppm <strong>of</strong> dissolved sulfur(Table 7.3). In <strong>the</strong> modelling, this 25ºC water is <strong>the</strong>n equilibrated with a rock similar in compositionto <strong>the</strong> Mount Neil Granite in <strong>the</strong> Mount Painter Inlier (Table 7.2, Coats and Blissett, 1971) at a 1:1weight ratio. Initial concentration <strong>of</strong> uranium in <strong>the</strong> granite is set at 50 ppm in <strong>the</strong> model. Theresults show that <strong>the</strong> oxygen saturated water at 25 o C dissolves all <strong>the</strong> available uranium, and allFe +2 -bearing silicates and oxides are converted to hematite. To model <strong>the</strong> downward flow <strong>of</strong> thisfluid into <strong>the</strong> granite and accompanying heating <strong>of</strong> <strong>the</strong> fluid due to <strong>the</strong> geo<strong>the</strong>rmal gradient, <strong>the</strong> fluidresulting from <strong>the</strong> initial reaction with granite reacts at equilibrium with <strong>the</strong> fresh granite atincreasing temperature up to 100 o C. The results show that <strong>the</strong> fluid which was oxidised at 25 o C anduranium rich is immediately reduced by <strong>the</strong> granite, and <strong>the</strong> uranium concentration in <strong>the</strong> fluid dropssignificantly (from 100 ppm to less than 1x10 9 ppm; Fig. 7.10). Thus <strong>the</strong> fluid moving downwardthrough <strong>the</strong> fresh granite cannot dissolve geologically significant concentrations <strong>of</strong> uranium. Only atvery high fluid rock ratios (<strong>of</strong> >1000) will <strong>the</strong> fluid in equilibrium with <strong>the</strong> granite be sufficientlyoxidised to carry significant concentrations <strong>of</strong> uranium. However, to attain such high fluid/rockratios, <strong>the</strong> fresh granite would be required to have unrealistically high permeability. Never<strong>the</strong>less,extremely high permeabilities may be possible in some wea<strong>the</strong>red zones and/or in breccia zones.Second, <strong>the</strong> chemical changes during upflow <strong>of</strong> <strong>the</strong> fluid in <strong>the</strong> circulation system are modelled. Theproperties <strong>of</strong> this fluid are initially set to be <strong>the</strong> same as those derived from <strong>the</strong> final interaction <strong>of</strong>down-flowing water with granite at 100 o C, modelled above. Upflow is modelled by cooling <strong>the</strong>fluid to 25 o C at rock-buffered conditions (in equilibrium with <strong>the</strong> granite, see Fig. 7.3). As <strong>the</strong> initialconcentration <strong>of</strong> uranium in <strong>the</strong> fluid is very low (