Uranium ore-forming systems of the - Geoscience Australia
Uranium ore-forming systems of the - Geoscience Australia
Uranium ore-forming systems of the - Geoscience Australia
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
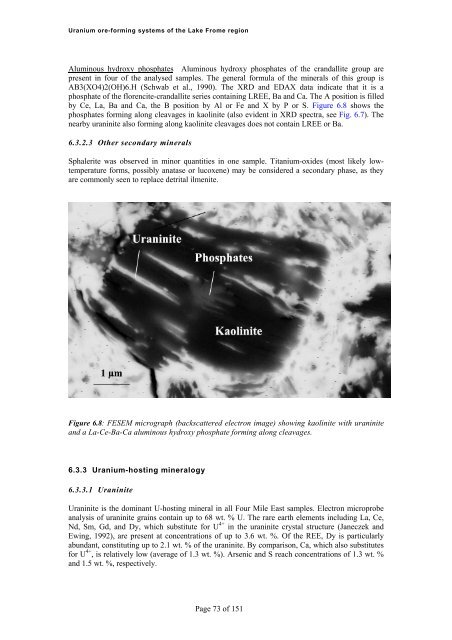
<strong>Uranium</strong> <strong>ore</strong>-<strong>forming</strong> <strong>systems</strong> <strong>of</strong> <strong>the</strong> Lake Frome regionAluminous hydroxy phosphates Aluminous hydroxy phosphates <strong>of</strong> <strong>the</strong> crandallite group arepresent in four <strong>of</strong> <strong>the</strong> analysed samples. The general formula <strong>of</strong> <strong>the</strong> minerals <strong>of</strong> this group isAB3(XO4)2(OH)6.H (Schwab et al., 1990). The XRD and EDAX data indicate that it is aphosphate <strong>of</strong> <strong>the</strong> fl<strong>ore</strong>ncite-crandallite series containing LREE, Ba and Ca. The A position is filledby Ce, La, Ba and Ca, <strong>the</strong> B position by Al or Fe and X by P or S. Figure 6.8 shows <strong>the</strong>phosphates <strong>forming</strong> along cleavages in kaolinite (also evident in XRD spectra, see Fig. 6.7). Thenearby uraninite also <strong>forming</strong> along kaolinite cleavages does not contain LREE or Ba.6.3.2.3 O<strong>the</strong>r secondary mineralsSphalerite was observed in minor quantities in one sample. Titanium-oxides (most likely lowtemperatureforms, possibly anatase or lucoxene) may be considered a secondary phase, as <strong>the</strong>yare commonly seen to replace detrital ilmenite.Figure 6.8: FESEM micrograph (backscattered electron image) showing kaolinite with uraniniteand a La-Ce-Ba-Ca aluminous hydroxy phosphate <strong>forming</strong> along cleavages.6.3.3 <strong>Uranium</strong>-hosting mineralogy6.3.3.1 UraniniteUraninite is <strong>the</strong> dominant U-hosting mineral in all Four Mile East samples. Electron microprobeanalysis <strong>of</strong> uraninite grains contain up to 68 wt. % U. The rare earth elements including La, Ce,Nd, Sm, Gd, and Dy, which substitute for U 4+ in <strong>the</strong> uraninite crystal structure (Janeczek andEwing, 1992), are present at concentrations <strong>of</strong> up to 3.6 wt. %. Of <strong>the</strong> REE, Dy is particularlyabundant, constituting up to 2.1 wt. % <strong>of</strong> <strong>the</strong> uraninite. By comparison, Ca, which also substitutesfor U 4+ , is relatively low (average <strong>of</strong> 1.3 wt. %). Arsenic and S reach concentrations <strong>of</strong> 1.3 wt. %and 1.5 wt. %, respectively.Page 73 <strong>of</strong> 151