Uranium ore-forming systems of the - Geoscience Australia
Uranium ore-forming systems of the - Geoscience Australia
Uranium ore-forming systems of the - Geoscience Australia
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
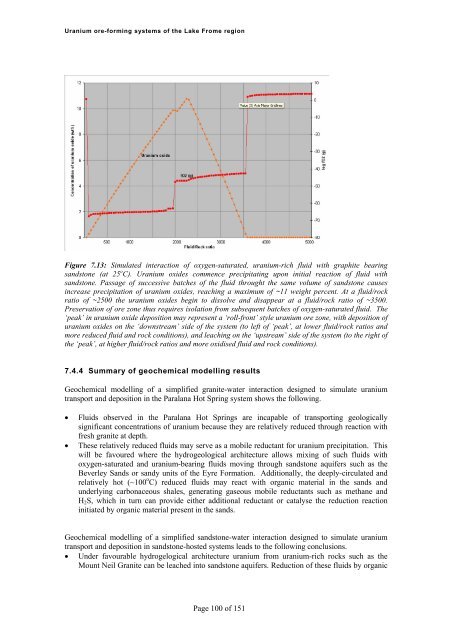
<strong>Uranium</strong> <strong>ore</strong>-<strong>forming</strong> <strong>systems</strong> <strong>of</strong> <strong>the</strong> Lake Frome regionFigure 7.13: Simulated interaction <strong>of</strong> oxygen-saturated, uranium-rich fluid with graphite bearingsandstone (at 25 o C). <strong>Uranium</strong> oxides commence precipitating upon initial reaction <strong>of</strong> fluid withsandstone. Passage <strong>of</strong> successive batches <strong>of</strong> <strong>the</strong> fluid throught <strong>the</strong> same volume <strong>of</strong> sandstone causesincrease precipitation <strong>of</strong> uranium oxides, reaching a maximum <strong>of</strong> ~11 weight percent. At a fluid/rockratio <strong>of</strong> ~2500 <strong>the</strong> uranium oxides begin to dissolve and disappear at a fluid/rock ratio <strong>of</strong> ~3500.Preservation <strong>of</strong> <strong>ore</strong> zone thus requires isolation from subsequent batches <strong>of</strong> oxygen-saturated fluid. The‘peak’ in uranium oxide deposition may represent a ‘roll-front’ style uranium <strong>ore</strong> zone, with deposition <strong>of</strong>uranium oxides on <strong>the</strong> ‘downstream’ side <strong>of</strong> <strong>the</strong> system (to left <strong>of</strong> ‘peak’, at lower fluid/rock ratios andm<strong>ore</strong> reduced fluid and rock conditions), and leaching on <strong>the</strong> ‘upstream’ side <strong>of</strong> <strong>the</strong> system (to <strong>the</strong> right <strong>of</strong><strong>the</strong> ‘peak’, at higher fluid/rock ratios and m<strong>ore</strong> oxidised fluid and rock conditions).7.4.4 Summary <strong>of</strong> geochemical modelling resultsGeochemical modelling <strong>of</strong> a simplified granite-water interaction designed to simulate uraniumtransport and deposition in <strong>the</strong> Paralana Hot Spring system shows <strong>the</strong> following.Fluids observed in <strong>the</strong> Paralana Hot Springs are incapable <strong>of</strong> transporting geologicallysignificant concentrations <strong>of</strong> uranium because <strong>the</strong>y are relatively reduced through reaction withfresh granite at depth.These relatively reduced fluids may serve as a mobile reductant for uranium precipitation. Thiswill be favoured where <strong>the</strong> hydrogeological architecture allows mixing <strong>of</strong> such fluids withoxygen-saturated and uranium-bearing fluids moving through sandstone aquifers such as <strong>the</strong>Beverley Sands or sandy units <strong>of</strong> <strong>the</strong> Eyre Formation. Additionally, <strong>the</strong> deeply-circulated andrelatively hot (~100 o C) reduced fluids may react with organic material in <strong>the</strong> sands andunderlying carbonaceous shales, generating gaseous mobile reductants such as methane andH 2 S, which in turn can provide ei<strong>the</strong>r additional reductant or catalyse <strong>the</strong> reduction reactioninitiated by organic material present in <strong>the</strong> sands.Geochemical modelling <strong>of</strong> a simplified sandstone-water interaction designed to simulate uraniumtransport and deposition in sandstone-hosted <strong>systems</strong> leads to <strong>the</strong> following conclusions. Under favourable hydrogelogical architecture uranium from uranium-rich rocks such as <strong>the</strong>Mount Neil Granite can be leached into sandstone aquifers. Reduction <strong>of</strong> <strong>the</strong>se fluids by organicPage 100 <strong>of</strong> 151