Uranium ore-forming systems of the - Geoscience Australia
Uranium ore-forming systems of the - Geoscience Australia
Uranium ore-forming systems of the - Geoscience Australia
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
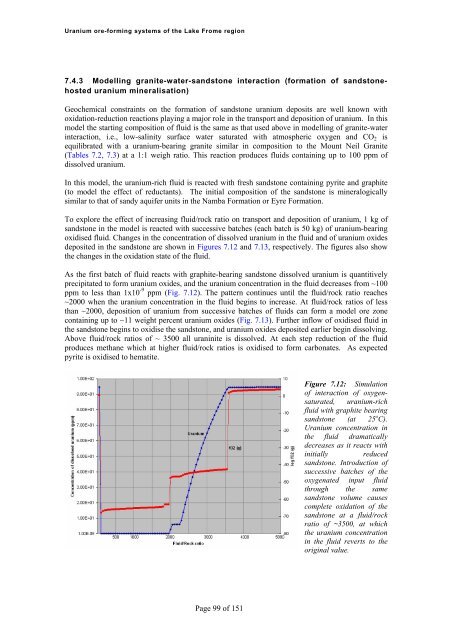
<strong>Uranium</strong> <strong>ore</strong>-<strong>forming</strong> <strong>systems</strong> <strong>of</strong> <strong>the</strong> Lake Frome region7.4.3 Modelling granite-water-sandstone interaction (formation <strong>of</strong> sandstonehosteduranium mineralisation)Geochemical constraints on <strong>the</strong> formation <strong>of</strong> sandstone uranium deposits are well known withoxidation-reduction reactions playing a major role in <strong>the</strong> transport and deposition <strong>of</strong> uranium. In thismodel <strong>the</strong> starting composition <strong>of</strong> fluid is <strong>the</strong> same as that used above in modelling <strong>of</strong> granite-waterinteraction, i.e., low-salinity surface water saturated with atmospheric oxygen and CO 2 isequilibrated with a uranium-bearing granite similar in composition to <strong>the</strong> Mount Neil Granite(Tables 7.2, 7.3) at a 1:1 weigh ratio. This reaction produces fluids containing up to 100 ppm <strong>of</strong>dissolved uranium.In this model, <strong>the</strong> uranium-rich fluid is reacted with fresh sandstone containing pyrite and graphite(to model <strong>the</strong> effect <strong>of</strong> reductants). The initial composition <strong>of</strong> <strong>the</strong> sandstone is mineralogicallysimilar to that <strong>of</strong> sandy aquifer units in <strong>the</strong> Namba Formation or Eyre Formation.To expl<strong>ore</strong> <strong>the</strong> effect <strong>of</strong> increasing fluid/rock ratio on transport and deposition <strong>of</strong> uranium, 1 kg <strong>of</strong>sandstone in <strong>the</strong> model is reacted with successive batches (each batch is 50 kg) <strong>of</strong> uranium-bearingoxidised fluid. Changes in <strong>the</strong> concentration <strong>of</strong> dissolved uranium in <strong>the</strong> fluid and <strong>of</strong> uranium oxidesdeposited in <strong>the</strong> sandstone are shown in Figures 7.12 and 7.13, respectively. The figures also show<strong>the</strong> changes in <strong>the</strong> oxidation state <strong>of</strong> <strong>the</strong> fluid.As <strong>the</strong> first batch <strong>of</strong> fluid reacts with graphite-bearing sandstone dissolved uranium is quantitivelyprecipitated to form uranium oxides, and <strong>the</strong> uranium concentration in <strong>the</strong> fluid decreases from ~100ppm to less than 1x10 -9 ppm (Fig. 7.12). The pattern continues until <strong>the</strong> fluid/rock ratio reaches~2000 when <strong>the</strong> uranium concentration in <strong>the</strong> fluid begins to increase. At fluid/rock ratios <strong>of</strong> lessthan ~2000, deposition <strong>of</strong> uranium from successive batches <strong>of</strong> fluids can form a model <strong>ore</strong> zonecontaining up to ~11 weight percent uranium oxides (Fig. 7.13). Fur<strong>the</strong>r inflow <strong>of</strong> oxidised fluid in<strong>the</strong> sandstone begins to oxidise <strong>the</strong> sandstone, and uranium oxides deposited earlier begin dissolving.Above fluid/rock ratios <strong>of</strong> ~ 3500 all uraninite is dissolved. At each step reduction <strong>of</strong> <strong>the</strong> fluidproduces methane which at higher fluid/rock ratios is oxidised to form carbonates. As expectedpyrite is oxidised to hematite.Figure 7.12: Simulation<strong>of</strong> interaction <strong>of</strong> oxygensaturated,uranium-richfluid with graphite bearingsandstone (at 25 o C).<strong>Uranium</strong> concentration in<strong>the</strong> fluid dramaticallydecreases as it reacts withinitially reducedsandstone. Introduction <strong>of</strong>successive batches <strong>of</strong> <strong>the</strong>oxygenated input fluidthrough <strong>the</strong> samesandstone volume causescomplete oxidation <strong>of</strong> <strong>the</strong>sandstone at a fluid/rockratio <strong>of</strong> ~3500, at which<strong>the</strong> uranium concentrationin <strong>the</strong> fluid reverts to <strong>the</strong>original value.Page 99 <strong>of</strong> 151