Uranium ore-forming systems of the - Geoscience Australia
Uranium ore-forming systems of the - Geoscience Australia
Uranium ore-forming systems of the - Geoscience Australia
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
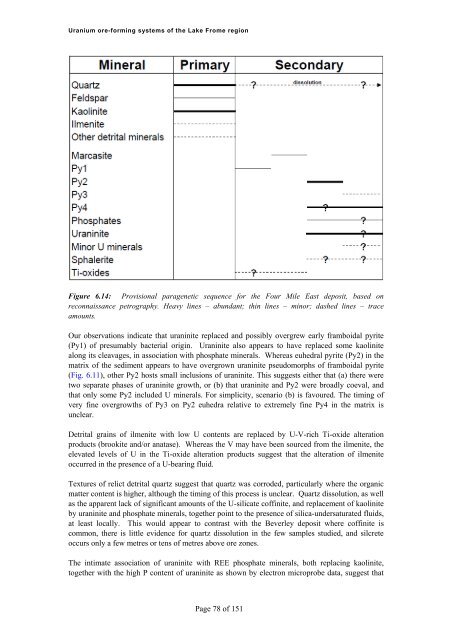
<strong>Uranium</strong> <strong>ore</strong>-<strong>forming</strong> <strong>systems</strong> <strong>of</strong> <strong>the</strong> Lake Frome regionFigure 6.14: Provisional paragenetic sequence for <strong>the</strong> Four Mile East deposit, based onreconnaissance petrography. Heavy lines – abundant; thin lines – minor; dashed lines – traceamounts.Our observations indicate that uraninite replaced and possibly overgrew early framboidal pyrite(Py1) <strong>of</strong> presumably bacterial origin. Uraninite also appears to have replaced some kaolinitealong its cleavages, in association with phosphate minerals. Whereas euhedral pyrite (Py2) in <strong>the</strong>matrix <strong>of</strong> <strong>the</strong> sediment appears to have overgrown uraninite pseudomorphs <strong>of</strong> framboidal pyrite(Fig. 6.11), o<strong>the</strong>r Py2 hosts small inclusions <strong>of</strong> uraninite. This suggests ei<strong>the</strong>r that (a) <strong>the</strong>re weretwo separate phases <strong>of</strong> uraninite growth, or (b) that uraninite and Py2 were broadly coeval, andthat only some Py2 included U minerals. For simplicity, scenario (b) is favoured. The timing <strong>of</strong>very fine overgrowths <strong>of</strong> Py3 on Py2 euhedra relative to extremely fine Py4 in <strong>the</strong> matrix isunclear.Detrital grains <strong>of</strong> ilmenite with low U contents are replaced by U-V-rich Ti-oxide alterationproducts (brookite and/or anatase). Whereas <strong>the</strong> V may have been sourced from <strong>the</strong> ilmenite, <strong>the</strong>elevated levels <strong>of</strong> U in <strong>the</strong> Ti-oxide alteration products suggest that <strong>the</strong> alteration <strong>of</strong> ilmeniteoccurred in <strong>the</strong> presence <strong>of</strong> a U-bearing fluid.Textures <strong>of</strong> relict detrital quartz suggest that quartz was corroded, particularly where <strong>the</strong> organicmatter content is higher, although <strong>the</strong> timing <strong>of</strong> this process is unclear. Quartz dissolution, as wellas <strong>the</strong> apparent lack <strong>of</strong> significant amounts <strong>of</strong> <strong>the</strong> U-silicate c<strong>of</strong>finite, and replacement <strong>of</strong> kaoliniteby uraninite and phosphate minerals, toge<strong>the</strong>r point to <strong>the</strong> presence <strong>of</strong> silica-undersaturated fluids,at least locally. This would appear to contrast with <strong>the</strong> Beverley deposit where c<strong>of</strong>finite iscommon, <strong>the</strong>re is little evidence for quartz dissolution in <strong>the</strong> few samples studied, and silcreteoccurs only a few metres or tens <strong>of</strong> metres above <strong>ore</strong> zones.The intimate association <strong>of</strong> uraninite with REE phosphate minerals, both replacing kaolinite,toge<strong>the</strong>r with <strong>the</strong> high P content <strong>of</strong> uraninite as shown by electron microprobe data, suggest thatPage 78 <strong>of</strong> 151