5 The role of quorum-sensing in the virulence of Pseudomonas ...
5 The role of quorum-sensing in the virulence of Pseudomonas ...
5 The role of quorum-sensing in the virulence of Pseudomonas ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
AQ: 32<br />
AQ: 33<br />
AQ: 34<br />
tapraid5/z9d-jid/z9d-jid/z9d01009/z9d4168d09a sangreyj S�5 3/20/09 Art: 42180<br />
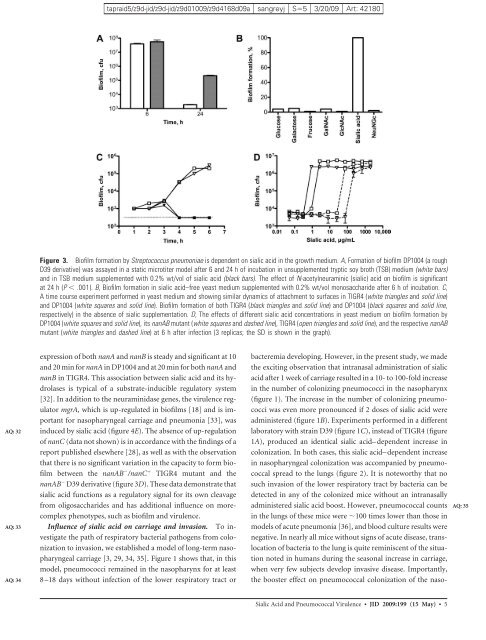
Figure 3. Bi<strong>of</strong>ilm formation by Streptococcus pneumoniae is dependent on sialic acid <strong>in</strong> <strong>the</strong> growth medium. A, Formation <strong>of</strong> bi<strong>of</strong>ilm DP1004 (a rough<br />
D39 derivative) was assayed <strong>in</strong> a static microtiter model after 6 and 24 h <strong>of</strong> <strong>in</strong>cubation <strong>in</strong> unsupplemented tryptic soy broth (TSB) medium (white bars)<br />
and <strong>in</strong> TSB medium supplemented with 0.2% wt/vol <strong>of</strong> sialic acid (black bars). <strong>The</strong> effect <strong>of</strong> N-acetylneuram<strong>in</strong>ic (sialic) acid on bi<strong>of</strong>ilm is significant<br />
at 24 h (P � .001). B, Bi<strong>of</strong>ilm formation <strong>in</strong> sialic acid–free yeast medium supplemented with 0.2% wt/vol monosaccharide after 6h<strong>of</strong><strong>in</strong>cubation. C,<br />
A time course experiment performed <strong>in</strong> yeast medium and show<strong>in</strong>g similar dynamics <strong>of</strong> attachment to surfaces <strong>in</strong> TIGR4 (white triangles and solid l<strong>in</strong>e)<br />
and DP1004 (white squares and solid l<strong>in</strong>e). Bi<strong>of</strong>ilm formation <strong>of</strong> both TIGR4 (black triangles and solid l<strong>in</strong>e) and DP1004 (black squares and solid l<strong>in</strong>e,<br />
respectively) <strong>in</strong> <strong>the</strong> absence <strong>of</strong> sialic supplementation. D, <strong>The</strong> effects <strong>of</strong> different sialic acid concentrations <strong>in</strong> yeast medium on bi<strong>of</strong>ilm formation by<br />
DP1004 (white squares and solid l<strong>in</strong>e), its nanAB mutant (white squares and dashed l<strong>in</strong>e), TIGR4 (open triangles and solid l<strong>in</strong>e), and <strong>the</strong> respective nanAB<br />
mutant (white triangles and dashed l<strong>in</strong>e) at 6 h after <strong>in</strong>fection (3 replicas; <strong>the</strong> SD is shown <strong>in</strong> <strong>the</strong> graph).<br />
expression <strong>of</strong> both nanA and nanB is steady and significant at 10<br />
and 20 m<strong>in</strong> for nanA <strong>in</strong> DP1004 and at 20 m<strong>in</strong> for both nanA and<br />
nanB <strong>in</strong> TIGR4. This association between sialic acid and its hydrolases<br />
is typical <strong>of</strong> a substrate-<strong>in</strong>ducible regulatory system<br />
[32]. In addition to <strong>the</strong> neuram<strong>in</strong>idase genes, <strong>the</strong> <strong>virulence</strong> regulator<br />
mgrA, which is up-regulated <strong>in</strong> bi<strong>of</strong>ilms [18] and is important<br />
for nasopharyngeal carriage and pneumonia [33], was<br />
<strong>in</strong>duced by sialic acid (figure 4E). <strong>The</strong> absence <strong>of</strong> up-regulation<br />
<strong>of</strong> nanC (data not shown) is <strong>in</strong> accordance with <strong>the</strong> f<strong>in</strong>d<strong>in</strong>gs <strong>of</strong> a<br />
report published elsewhere [28], as well as with <strong>the</strong> observation<br />
that <strong>the</strong>re is no significant variation <strong>in</strong> <strong>the</strong> capacity to form bi<strong>of</strong>ilm<br />
between <strong>the</strong> nanAB � /nanC � TIGR4 mutant and <strong>the</strong><br />
nanAB � D39 derivative (figure 3D). <strong>The</strong>se data demonstrate that<br />
sialic acid functions as a regulatory signal for its own cleavage<br />
from oligosaccharides and has additional <strong>in</strong>fluence on morecomplex<br />
phenotypes, such as bi<strong>of</strong>ilm and <strong>virulence</strong>.<br />
Influence <strong>of</strong> sialic acid on carriage and <strong>in</strong>vasion. To <strong>in</strong>vestigate<br />
<strong>the</strong> path <strong>of</strong> respiratory bacterial pathogens from colonization<br />
to <strong>in</strong>vasion, we established a model <strong>of</strong> long-term nasopharyngeal<br />
carriage [3, 29, 34, 35]. Figure 1 shows that, <strong>in</strong> this<br />
model, pneumococci rema<strong>in</strong>ed <strong>in</strong> <strong>the</strong> nasopharynx for at least<br />
8–18 days without <strong>in</strong>fection <strong>of</strong> <strong>the</strong> lower respiratory tract or<br />
bacteremia develop<strong>in</strong>g. However, <strong>in</strong> <strong>the</strong> present study, we made<br />
<strong>the</strong> excit<strong>in</strong>g observation that <strong>in</strong>tranasal adm<strong>in</strong>istration <strong>of</strong> sialic<br />
acid after 1 week <strong>of</strong> carriage resulted <strong>in</strong> a 10- to 100-fold <strong>in</strong>crease<br />
<strong>in</strong> <strong>the</strong> number <strong>of</strong> coloniz<strong>in</strong>g pneumococci <strong>in</strong> <strong>the</strong> nasopharynx<br />
(figure 1). <strong>The</strong> <strong>in</strong>crease <strong>in</strong> <strong>the</strong> number <strong>of</strong> coloniz<strong>in</strong>g pneumococci<br />
was even more pronounced if 2 doses <strong>of</strong> sialic acid were<br />
adm<strong>in</strong>istered (figure 1B). Experiments performed <strong>in</strong> a different<br />
laboratory with stra<strong>in</strong> D39 (figure 1C), <strong>in</strong>stead <strong>of</strong> TIGR4 (figure<br />
1A), produced an identical sialic acid–dependent <strong>in</strong>crease <strong>in</strong><br />
colonization. In both cases, this sialic acid–dependent <strong>in</strong>crease<br />
<strong>in</strong> nasopharyngeal colonization was accompanied by pneumococcal<br />
spread to <strong>the</strong> lungs (figure 2). It is noteworthy that no<br />
such <strong>in</strong>vasion <strong>of</strong> <strong>the</strong> lower respiratory tract by bacteria can be<br />
detected <strong>in</strong> any <strong>of</strong> <strong>the</strong> colonized mice without an <strong>in</strong>tranasally<br />
adm<strong>in</strong>istered sialic acid boost. However, pneumococcal counts<br />
<strong>in</strong> <strong>the</strong> lungs <strong>of</strong> <strong>the</strong>se mice were �100 times lower than those <strong>in</strong><br />
models <strong>of</strong> acute pneumonia [36], and blood culture results were<br />
negative. In nearly all mice without signs <strong>of</strong> acute disease, translocation<br />
<strong>of</strong> bacteria to <strong>the</strong> lung is quite rem<strong>in</strong>iscent <strong>of</strong> <strong>the</strong> situation<br />
noted <strong>in</strong> humans dur<strong>in</strong>g <strong>the</strong> seasonal <strong>in</strong>crease <strong>in</strong> carriage,<br />
when very few subjects develop <strong>in</strong>vasive disease. Importantly,<br />
<strong>the</strong> booster effect on pneumococcal colonization <strong>of</strong> <strong>the</strong> naso-<br />
Sialic Acid and Pneumococcal Virulence ● JID 2009:199 (15 May) ● 5<br />
AQ: 35