PHYSICS
n - susliks.lv
n - susliks.lv
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Control Works<br />
Modulus 4<br />
Acoustics, Bioacoustics, Acoustobiology<br />
Problem. Estimate the intensity of sound which causes pain using<br />
Table 6.1.<br />
Answer: 10 W1m 2 •<br />
Problem. A point source emits sound waves with a power of 80 W.<br />
Find the intensity at a distance 3 m from the source.<br />
Answer: 0.707 W/m 2 •<br />
Problem. The bats of the Vespertilionidae family emit short high<br />
frequency pulses. Find the time of echo arrival after emission if the<br />
distance between the bat and insect is 10m.<br />
Answer: 58.8 ms.<br />
Example. Doppler ultrasound technique is able to measure blood<br />
velocity. If an artery has a typical blood velocity (about 1 m/s), what is<br />
the magnitude of the Doppler shift, if the velocity of ultrasound in the<br />
blood is 1500 m/s and the frequency is 5 MHz?<br />
Answer: 6.7 kHz.<br />
Animals Extremes!<br />
The sensitivity of cockroaches (Blattodeae) to vibrations is<br />
10- 8 em - the amplitude of these vibrations is equal to the diameter<br />
of hydrogen atom.<br />
Most Powerful Sound - Finback whale (Balaenoptera) <br />
10 W, within a range of 10000 km.<br />
Best Hearing - Barn Owl (Tyto alba) and Great Horned Owl<br />
(Bubo virginianusi,<br />
Chapter 10. MOLECULAR <strong>PHYSICS</strong><br />
UllIn. _liM ., _w, • Ili~<br />
10.1. IDEAL GAS<br />
p V = nRT (10.1)<br />
where n = m/M is the number of moles, m is mass of gas, M is<br />
molecular weight of the substance (gjmole), and R is the universal<br />
gas constant (8.31 Jjmol·K = 0.0821 Latrn/rnol-K).<br />
The previous equation can be written as:<br />
p V = NkT (10.2)<br />
where N is the total number of molecules, and k the Boltzmann's<br />
constant (1.38.10- 23 JjK).<br />
10.2. REAL GAS<br />
Real gases exhibit properties contrary to the ideal gas law; for<br />
example, real gas particles have a finite volume and measurable<br />
intermolecular forces. These two effects can be incorporated into<br />
a modified van der Waals' equation as:<br />
a<br />
(p + -2) (V - b) = RT (10.3)<br />
V<br />
where a and b are empirical constants.<br />
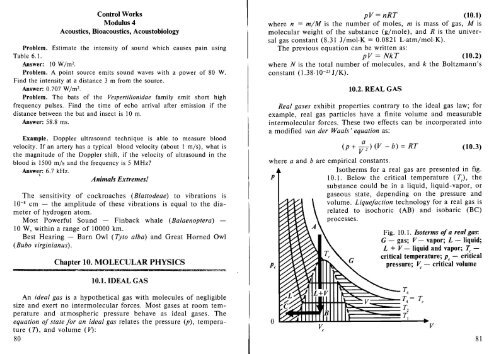
Isotherms for a real gas are presented in fig.<br />
P I<br />
10.1. Below the critical temperature (Te>, the<br />
substance could be in a liquid, liquid-vapor, or<br />
Pc<br />
gaseous state, depending on the pressure and<br />
volume. Liquefaction technology for a real gas is<br />
related to isochoric (AB) and isobaric (Be)<br />
processes.<br />
Fig. 10.1. Isoterms ofa real gas:<br />
G - gas; V vapor; L - liquid;<br />
L + V liquid and vapor; I: <br />
critical temperature; Pc - critical<br />
pressure; ~ - critical volume<br />
An ideal gas is a hypothetical gas with molecules of negligible<br />
size and exert no intermolecular forces. Most gases at room temperature<br />
and atmospheric pressure behave as ideal gases. The<br />
equation of state for an ideal gas relates the pressure (p), temperature<br />
(1), and volume (V):<br />
80<br />
o<br />
/)11111111111'1>:""""= V<br />
~<br />
T 4<br />
T=T<br />
4 c<br />
T 2<br />
T 1<br />
v<br />
81