PHYSICS
n - susliks.lv
n - susliks.lv
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
A polarimeter is a device that uses optical activity to measure<br />
the angle of rotation of polarized light. It consists of an elongated<br />
optical cell mounted between two polarizing lenses, one of which<br />
is fixed (polarizer) and the other being free to rotate (analyzer).<br />
The unpolarized light that is passing through the polarizer is<br />
polarized; the analyser intercepts this polarised light beam with<br />
its transmission axis at an angle qJ to the axis of the polyrizer. This<br />
angle qJ is measured by the registering system (fig. 21.16).<br />
21.4. QUANTUM OPTICS<br />
21.4.1. Photoelectric Effect<br />
In the latter part of the 19 th century, experiments showed that<br />
when light is incident on certain metallic surfaces, electrons are<br />
emitted from the surfaces. This phenomenon is known as the<br />
photoelectric effect could not be understood within the framework<br />
of classical physics.<br />
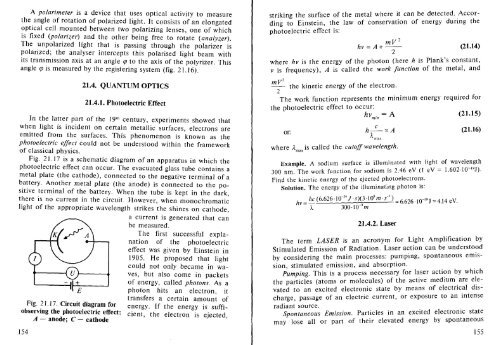
Fig. 21.17 is a schematic diagram of an apparatus in which the<br />
photoelectric effect can occur. The evacuated glass tube contains a<br />
metal plate (the cathode), connected to the negative terminal of a<br />
battery. Another metal plate (the anode) is connected to the positive<br />
terminal of the battery. When the tube is kept in the dark,<br />
there is no current in the circuit. However, when monochromatic<br />
light of the appropriate wavelength strikes the shines on cathode,<br />
a current is generated that can<br />
be measured.<br />
Fig. 21.17. Circuit diagram for<br />
observing the photoelectric effect:<br />
A - anode; C - cathode<br />
The first successful explanation<br />
of the photoelectric<br />
effect was given by Einstein in<br />
1905. He proposed that light<br />
could not only became in waves,<br />
but also come in packets<br />
of energy, called photons. As a<br />
photon hits an electron, it<br />
transfers a certain amount of<br />
energy. If the energy is sufficient,<br />
the electron is ejected,<br />
striking the surface of the metal where it can be detected. According<br />
to Einstein, the law of conservation of energy during the<br />
photoelectric effect is:<br />
mV 2<br />
hv = A +-- (21.14)<br />
2<br />
where hv is the energy of the photon (here h is Plank's constant,<br />
v is frequency), A is called the work function of the metal, and<br />
m;2<br />
the kinetic energy of the electron.<br />
The work function represents the minimum energy required for<br />
the photoelectric effect to occur:<br />
hv . = A<br />
min<br />
(21.15)<br />
or:<br />
c<br />
h-=A<br />
Am"'<br />
(21.16)<br />
where A is called the cutoff wavelength.<br />
max<br />
Example. A sodium surface is illuminated with light of wavelength<br />
300 nm. The work function for sodium is 2.46 eV (I eV = 1.602.10- 191).<br />
Find the kinetic energy of the ejected photoelectrons.<br />
Solution. The energy of the illuminating photon is:<br />
34 8<br />
hv =he (6.626.10- J ·s)(3·10 m 'S-I) =6.626 .10- 19 J =4.14 eV.<br />
A. 300·1O- 9m<br />
21.4.2. Laser<br />
The term LASER is an acronym for Light Amplification by<br />
Stimulated Emission of Radiation. Laser action can be understood<br />
by considering the main processes: pumping, spontaneous emission,<br />
stimulated emission, and absorption.<br />
Pumping. This is a process necessary for laser action by which<br />
the particles (atoms or molecules) of the active medium are elevated<br />
to an excited electronic state by means of electrical discharge,<br />
passage of an electric current, or exposure to an intense<br />
radiant source.<br />
Spontaneous Emission. Particles in an excited electronic state<br />
may lose all or part of their elevated energy by spontaneous<br />
154 155