PHYSICS
n - susliks.lv
n - susliks.lv
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
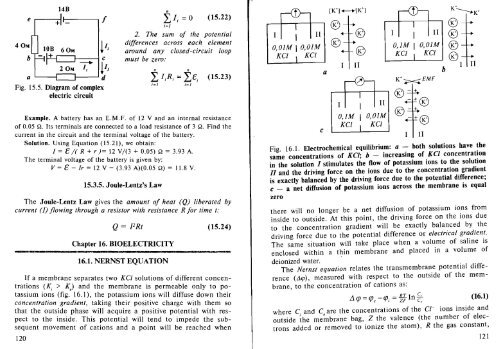
14B<br />
e i .11 if<br />
40MI I lOB 60M ~I2<br />
b~+<br />
a '----I<br />
C<br />
2 OM ~ ~IJ<br />
Fig. 15.5. Diagram of complex<br />
electric circuit<br />
d<br />
n<br />
LI; = 0<br />
;;]<br />
(15.22)<br />
2. The sum of the potential<br />
differences across each element<br />
around any closed-circuit loop<br />
must be zero:<br />
n<br />
n<br />
L t«, = LE; (15.23)<br />
;;} ;;}<br />
Example. A battery has an E.M.F. of 12 V and an internal resistance<br />
of 0.05 Q. Its terminals are connected to a load resistance of 3 Q. Find the<br />
current in the circuit and the terminal voltage of the battery.<br />
Solution. Using Equation (15.21), we obtain:<br />
J = E /( R + r )= 12 V/(3 + 0.05) Q = 3.93 A.<br />
The terminal voltage of the battery is given by:<br />
V = E - Jr = 12 V - (3.93 A)(0.05 Q) = 11.8 V.<br />
15.3.5. Joule-Lentz's Law<br />
The Joule-Lentz Law gives the amount of heat (Q) liberated by<br />
current (f) flowing through a resistor with resistance R for time t:<br />
Q = J2Rt<br />
Chapter 16. BIOELECTRICITY<br />
(15.24)<br />
,., •• wn _Ii i ~ J II lMli::la....,qlllllfJllt\<br />
16.1. NERNST EQUATION<br />
If a membrane separates two KCI solutions of different concentrations<br />
(K; > K.) and the membrane is permeable only to potassium<br />
ions (fig. 16.1), the potassium ions will diffuse down their<br />
concentration gradient, taking their positive charge with them so<br />
that the outside phase will acquire a positive potential with respect<br />
to the inside. This potential will tend to impede the subsequent<br />
movement of cations and a point will be reached when<br />
120<br />
I I I I II<br />
O,OlM I O,OlM<br />
KCI . KCI<br />
c<br />
a<br />
[K+]....-[K+]<br />
@<br />
@<br />
I<br />
I<br />
O,lM<br />
KCI<br />
@<br />
@<br />
II<br />
II<br />
O,OlM<br />
KCI<br />
I .<br />
O,lM<br />
KCI<br />
II<br />
O,OlM<br />
KCI<br />
~~~+ EMF<br />
-+@<br />
@-+<br />
-+@<br />
I I II<br />
b<br />
K'------. +<br />
~K<br />
@<br />
@<br />
@<br />
@I I II<br />
Fig. 16.1. Electrochemical equilibrium: a - both solutions have the<br />
same concentrations of KCf; b - increasing of Kef concentration<br />
in the solution I stimulates the flow of potassium ions to the solution<br />
II and the driving force on the ions due to the concentration gradient<br />
is exactly balanced by the driving force due to the potential difference;<br />
c - a net diffusion of potassium ions across the membrane is equal<br />
zero<br />
there will no longer be a net diffusion of potassium ions from<br />
inside to outside. At this point, the driving force on the ions due<br />
to the concentration gradient will be exactly balanced by the<br />
driving force due to the potential difference or electrical gradient.<br />
The same situation will take place when a volume of saline is<br />
enclosed within a thin membrane and placed in a volume of<br />
deionized water. .<br />
The Nernst equation relates the transmembrane potential difference<br />
(L1