Asia Personal Care & Cosmetics Market Guide 2016
AsiaCosmeticsMarketGuide
AsiaCosmeticsMarketGuide
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
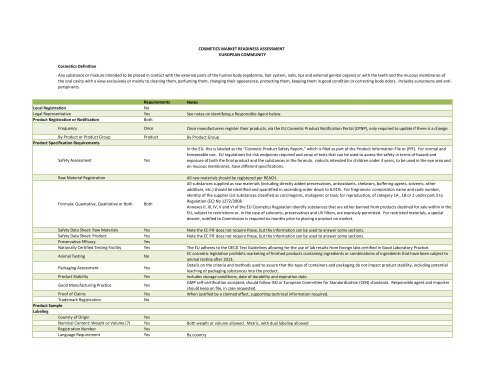
COSMETICS MARKET READINESS ASSESSMENT<br />
EUROPEAN COMMUNITY<br />
<strong>Cosmetics</strong> Definition<br />
Any substance or mixture intended to be placed in contact with the external parts of the human body (epidermis, hair system, nails, lips and external genital organs) or with the teeth and the mucous membranes of<br />
the oral cavity with a view exclusively or mainly to cleaning them, perfuming them, changing their appearance, protecting them, keeping them in good condition or correcting body odors. Includes sunscreens and antiperspirants.<br />
Requirements Notes<br />
Local Registration<br />
No<br />
Legal Representative Yes See notes on identifying a Responsible Agent below.<br />
Product Registration or Notification<br />
Both<br />
Frequency Once Once manufacturers register their products, via the EU Cosmetic Product Notification Portal (CPNP), only required to update if there is a change.<br />
By Product or Product Group Product By Product Group<br />
Product Specification Requirements<br />
Safety Assessment<br />
Yes<br />
In the EU, this is labeled as the “Cosmetic Product Safety Report,” which is filed as part of the Product Information File or (PIF). For normal and<br />
foreseeable use. EU regulations list risk endpoints required and array of tests that can be used to assess the safety in terms of hazard and<br />
exposure of both the final product and the substances in the formula. roducts intended for children under 3 years, to be used in the eye area and<br />
on mucous membranes, have different specifications.<br />
Raw Material Registration<br />
Formula: Quantative, Qualitative or Both<br />
Both<br />
All raw materials should be registered per REACH.<br />
All substances supplied as raw materials (including directly added preservatives, antioxidants, chelators, buffering agents, solvents, other<br />
additives, etc.) should be identified and quantified in ascending order down to 0.01%. For fragrances: composition name and code number,<br />
identity of the supplier List substances classified as carcinogenic, mutagenic or toxic for reproduction, of category 1A , 1B or 2 under part 3 to<br />
Regulation (EC) No 1272/2008<br />
Annexes II, III, IV, V and VI of the EU <strong>Cosmetics</strong> Regulation identify substances that are either banned from products destined for sale within in the<br />
EU, subject to restrictions or, in the case of colorants, preservatives and UV filters, are expressly permitted. For restricted materials, a special<br />
dossier, notified to Commission is required six months prior to placing a product on market.<br />
Safety Data Sheet: Raw Materials Yes Note the EC PIF does not require these, but the information can be used to answer some sections.<br />
Safety Data Sheet: Product Yes Note the EC PIF does not require these, but the information can be used to answer some sections.<br />
Preservative Efficacy<br />
Yes<br />
Nationally Certified Testing Facility Yes The EU adheres to the OECD Test <strong>Guide</strong>lines allowing for the use of lab results from foreign labs certified in Good Laboratory Practice.<br />
Animal Testing<br />
No<br />
EC cosmetic legislation prohibits marketing of finished products containing ingredients or combinations of ingredients that have been subject to<br />
animal testing after 2013.<br />
Packaging Assessment<br />
Yes<br />
Details on the criteria and methods used to assure that the type of containers and packaging do not impact product stability, including potential<br />
leaching of packaging substances into the product.<br />
Product Stability Yes Includes storage conditions, date of durability and expiration date.<br />
Good Manufacturing Practice<br />
Yes<br />
GMP self-certification accepted, should follow ISO or European Committee for Standardization (CEN) standards. Responsible agent and importer<br />
should keep on file, in case requested.<br />
Proof of Claims Yes When justified by a claimed effect, supporting technical information required.<br />
Trademark Registration<br />
No<br />
Product Sample<br />
Labeling<br />
Country of Origin<br />
Yes<br />
Nominal Content: Weight or Volume (?) Yes Both weight or volume allowed. Metric, with dual labeling allowed<br />
Registration Number<br />
Yes<br />
Language Requirement Yes By country