Darstellung und Charakterisierung neuer niedrigkoordinierter ...
Darstellung und Charakterisierung neuer niedrigkoordinierter ...
Darstellung und Charakterisierung neuer niedrigkoordinierter ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Me<br />
Me<br />
Me<br />
Si<br />
Si<br />
N<br />
Me<br />
Me<br />
Me<br />
As<br />
Cl<br />
Scheme 4. Suggested reaction mechanism of the GaCl3-assisted methyl/chlorine exchange in bis(trimethylsilyl)amino(dichloro)arsane (3), yielding cyclodisilazane<br />
(4) via release of Me2AsCl.<br />
Me 3Si–N 3 to give a tetrazarsole (m-terphenyl 6d = Ar # = 2,6-Mes 2-<br />
C 6H 3, Mes = 2,4,6-Me 3C 6H 2). 3 This at least was the idea which<br />
worked well for the analog phosphorus compo<strong>und</strong> but not for 5. 2f<br />
Upon adding a solution of GaCl 3 in CH 2Cl 2 to a solution of 5 in<br />
CH 2Cl 2 the formation of an iminoarsane was not observed but<br />
again a chlorine/methyl exchange reaction occurred as displayed<br />
by 29 Si, 1 H and 13 C NMR studies. Recrystallization from toluene<br />
gave crystals suitable for an X-ray structure determination<br />
revealing again a methyl/chlorine exchange product, now with<br />
two chlorine atoms attached to the silicon and a dimethylarsane<br />
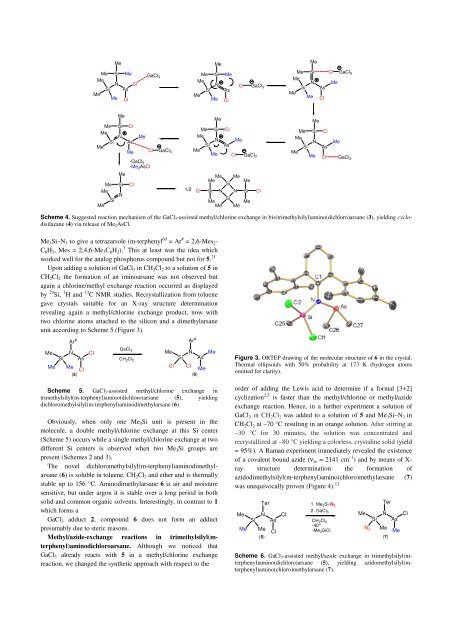
unit according to Scheme 5 (Figure 3).<br />
Ar #<br />
Me<br />
Me<br />
Me<br />
Si<br />
Me<br />
Me<br />
Me<br />
Si<br />
Me<br />
Si<br />
N<br />
Me<br />
Si<br />
N<br />
Cl<br />
As<br />
Me<br />
Scheme 5. GaCl3-assisted methyl/chlorine exchange in<br />
trimethylsilyl(m-terphenyl)amino(dichloro)arsane (5), yielding<br />
dichloromethylsilyl(m-terphenyl)aminodimethylarsane (6).<br />
Cl<br />
GaCl 3<br />
Obviously, when only one Me 3Si unit is present in the<br />
molecule, a double methyl/chlorine exchange at this Si center<br />
(Scheme 5) occurs while a single methyl/chlorine exchange at two<br />
different Si centers is observed when two Me 3Si groups are<br />
present (Schemes 2 and 3).<br />
The novel dichloromethylsilyl(m-terphenyl)aminodimethylarsane<br />
(6) is soluble in toluene, CH 2Cl 2 and ether and is thermally<br />
stable up to 156 °C. Aminodimethylarsane 6 is air and moisture<br />
sensitive, but <strong>und</strong>er argon it is stable over a long period in both<br />
solid and common organic solvents. Interestingly, in contrast to 1<br />
which forms a<br />
GaCl3 adduct 2, compo<strong>und</strong> 6 does not form an adduct<br />
presumably due to steric reasons.<br />
Methyl/azide-exchange reactions in trimethylsilyl(mterphenyl)aminodichloroarsane.<br />
Although we noticed that<br />
GaCl 3 already reacts with 5 in a methyl/chlorine exchange<br />
reaction, we changed the synthetic approach with respect to the<br />
Cl<br />
Me<br />
GaCl 3<br />
Cl<br />
-GaCl 3<br />
-Me 2AsCl<br />
GaCl 3<br />
Me<br />
Me<br />
Me<br />
Cl<br />
Si<br />
Me<br />
Me<br />
Me<br />
Si<br />
Me Me<br />
Me Me<br />
Si<br />
Si<br />
Si<br />
N<br />
Me<br />
Me<br />
Si<br />
N<br />
Me<br />
Me<br />
N<br />
Me<br />
Me<br />
Me<br />
Si<br />
N<br />
As<br />
Cl<br />
CH2Cl2 Me<br />
Si<br />
N<br />
As<br />
Me<br />
Me Me<br />
Cl<br />
(5)<br />
Cl Cl<br />
Me<br />
(6)<br />
1/2<br />
Ar #<br />
Me<br />
As<br />
Cl<br />
Cl<br />
As<br />
Si<br />
Cl<br />
N<br />
Me<br />
Cl<br />
Me<br />
GaCl 3<br />
Si<br />
Me<br />
GaCl 3<br />
Cl<br />
Me<br />
Me<br />
Me<br />
Si<br />
Me<br />
Me<br />
Me<br />
Si<br />
Si<br />
N<br />
Me<br />
Me<br />
Me<br />
Si<br />
N<br />
As<br />
Cl<br />
Cl<br />
As<br />
GaCl 3<br />
Figure 3. ORTEP drawing of the molecular structure of 6 in the crystal.<br />
Thermal ellipsoids with 50% probability at 173 K (hydrogen atoms<br />
omitted for clarity).<br />
order of adding the Lewis acid to determine if a formal [3+2]<br />
cyclization 2,3 is faster than the methyl/chlorine or methyl/azide<br />
exchange reaction. Hence, in a further experiment a solution of<br />
GaCl 3 in CH 2Cl 2 was added to a solution of 5 and Me 3Si–N 3 in<br />
CH 2Cl 2 at –70 °C resulting in an orange solution. After stirring at<br />
–30 °C for 30 minutes, the solution was concentrated and<br />
recrystallized at –80 °C yielding a colorless, crystaline solid (yield<br />
= 95%). A Raman experiment immediately revealed the existence<br />
of a covalent bo<strong>und</strong> azide (νas = 2141 cm –1 ) and by means of Xray<br />
structure determination the formation of<br />
azidodimethylsilyl(m-terphenyl)amino(chloro)methylarsane (7)<br />
was unequivocally proven (Figure 4). 12<br />
Scheme 6. GaCl3-assisted methyl/azide exchange in trimethylsilyl(mterphenyl)amino(dichloro)arsane<br />
(5), yielding azidomethylsilyl(mterphenyl)amino(chloro)methylarsane<br />
(7).<br />
Cl<br />
Me<br />
Me<br />
Me Cl GaCl3 Ter<br />
1. Me3Si-N3 Ter<br />
Me<br />
Me<br />
N<br />
Si<br />
Me<br />
As<br />
Cl<br />
Cl<br />
2. GaCl3 CH2Cl2 -40°<br />
-Me3SiCl Me N<br />
Si<br />
N3 Me<br />
Cl<br />
As<br />
Me<br />
(5) (7)