Richtlijn Sterilisatie van de vrouw - NVOG
Richtlijn Sterilisatie van de vrouw - NVOG
Richtlijn Sterilisatie van de vrouw - NVOG
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
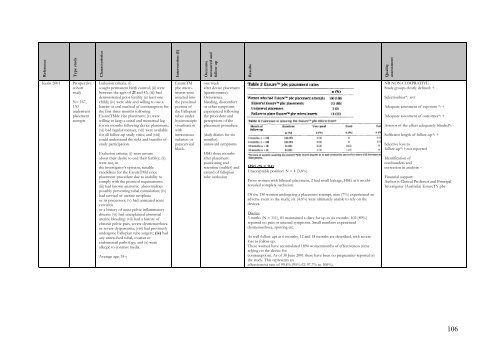
ReferenceType studyCharacteristicsIntervention (I)Outcomemeasured andfollow upResultsQualityassessmentKerin 2001ProspectivecohortstudyN= 167,130un<strong>de</strong>rwentplacementatemptsInclusion criteria: (i)sought permanent birth control; (ii) werebetween the ages of 21 and 43; (iii) had<strong>de</strong>monstrated prior fertility (at least onechild); (iv) were able and willing to use abarrier or oral method of contraception forthe first three months followingEssureTM<strong>de</strong> vice placement; (v) werewilling to keep a coital and menstrual logfor six months following <strong>de</strong>vice placement;(vi) had regular menses, (vii) were availablefor all follow-up study visits; and (viii)could un<strong>de</strong>rstand the risks and benefits ofstudy participation.Exclusion criteria: (i) were unsureabout their <strong>de</strong>sire to end their fertility; (ii)were not, inthe investigator’s opinion, suitablecandidates for the EssureTMd eviceplacement procedure due to inability tocomply with the protocol requirements:(iii) had known anatomic abnormalitiespossibly preventing tubal cannulation; (iv)had cervical or uterine neoplasiaor its precursors; (v) had untreated acutecervicitisor a history of acute pelvic inflammatorydisease: (vi) had unexplained abnormaluterine bleeding: (vii) had a history ofchronic pelvic pain, severe dysmenorrhoeaor severe dyspareunia; (viii) had previouslyun<strong>de</strong>rgone Fallopian tube surgery; (ix) hadany unresolved tubal, ovarian orendometrial pathology; and (x) wereallergic to contrast media.Average age: 35 yEssureTMpbc microinsertswereinserted intothe proximalportion ofthe Fallopiantubes un<strong>de</strong>rhysteroscopicvisualisationwithintravenoussedation orparacervicalblock.one weekafter <strong>de</strong>vice placement(questionnaire):Occurrencebleeding, discomfortor other symptomsexperienced followingthe procedure andperceptions of theplacement procedure.(daily diaries for sixmonths):untoward symptomsHSG three monthsafter placement:positioning andretention (stable?) an<strong>de</strong>xtend of fallopiantube occlusionHSG (N = 114)Unacceptable position: N = 4 (3.6%).From women with bilateral placement, 2 had small leakage, HSG at 6 mothsrevealed complete occlusion.Of the 130 women un<strong>de</strong>rgoing a placement attempt, nine (7%) experienced anadverse event in the study; six (4.6%) were ultimately unable to rely on the<strong>de</strong>vices.Diaries:3 mnths (N = 111), 81 maintained a diary for up to six months. 102 (89%)reported no pain or unusual symptoms. Small numbers experienceddysmenorrhoea, spotting etc.As well follow ups at 6 months, 12 and 18 months are <strong>de</strong>scribed, with severeloss to follow up.These women have accumulated 1894 womenmonths of effectiveness (timerelying on the <strong>de</strong>vice forcontraception). As of 30 June 2001 there have been no pregnancies reported inthe study. This represents aneffectiveness rate of 99.4% (95% CI 97.7% to 100%).NB NON-COMPRATIVEStudy groups clearly <strong>de</strong>fined: +Selectionbias*: nvtA<strong>de</strong>quate assesment of exposure *: +A<strong>de</strong>quate assesment of outcomes*: +Assesor of the effect a<strong>de</strong>quately blin<strong>de</strong>d*: -Sufficient length of follow-up*: +Selective loss tofollow-up*: ? not reportedI<strong>de</strong>ntification ofconfoun<strong>de</strong>rs andcorrection in analysis: -Financial support:Author is Clinical Professor and PrincipalInvestigator (Australia) EssureTY pbc106