Richtlijn Sterilisatie van de vrouw - NVOG
Richtlijn Sterilisatie van de vrouw - NVOG
Richtlijn Sterilisatie van de vrouw - NVOG
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
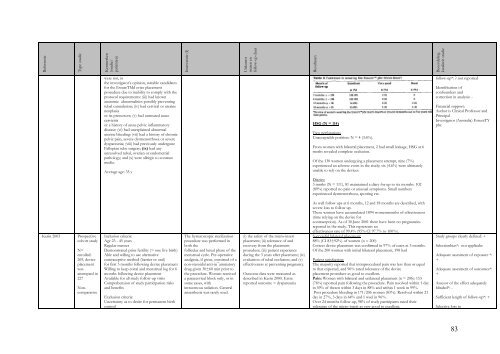
ReferentieType studieKenmerken(studie/patiënten)Interventie (I)Uitkomstmaten enfollow-up duurResultatenBeoor<strong>de</strong>lingkwaliteit studiewere not, inthe investigator’s opinion, suitable candidatesfor the EssureTMd evice placementprocedure due to inability to comply with theprotocol requirements: (iii) had knownanatomic abnormalities possibly preventingtubal cannulation; (iv) had cervical or uterineneoplasiaor its precursors; (v) had untreated acutecervicitisor a history of acute pelvic inflammatorydisease: (vi) had unexplained abnormaluterine bleeding: (vii) had a history of chronicpelvic pain, severe dysmenorrhoea or severedyspareunia; (viii) had previously un<strong>de</strong>rgoneFallopian tube surgery; (ix) had anyunresolved tubal, ovarian or endometrialpathology; and (x) were allergic to contrastmedia.Average age: 35 yHSG (N = 114)Two perforationsUnacceptable position: N = 4 (3.6%).From women with bilateral placement, 2 had small leakage, HSG at 6moths revealed complete occlusion.Of the 130 women un<strong>de</strong>rgoing a placement attempt, nine (7%)experienced an adverse event in the study; six (4.6%) were ultimatelyunable to rely on the <strong>de</strong>vices.follow-up*: ? not reportedI<strong>de</strong>ntification ofconfoun<strong>de</strong>rs andcorrection in analysis: -Financial support:Author is Clinical Professor andPrincipalInvestigator (Australia) EssureTYpbcDiaries:3 mnths (N = 111), 81 maintained a diary for up to six months. 102(89%) reported no pain or unusual symptoms. Small numbersexperienced dysmenorrhoea, spotting etc.Kerin 2003Prospectivecohort studyN=enrolled:269, <strong>de</strong>viceplacementwasattempted in227NoncomparativeInclusion criteria:Age 23 - 45 yearsRegular mensesDemonstrated prior fertility (> one live birth)Able and willing to use alternativecontraceptive method (barrier or oral)for first 3 months following <strong>de</strong>vice placementWilling to keep coital and menstrual log for 6months following <strong>de</strong>vice placementAvailable for all study follow-up visitsComprehension of study participation risksand benefitsExclusion criteria:Uncertainty as to <strong>de</strong>sire for permanent birthcontrolThe hysteroscopic sterilizationprocedure was performed inboth thefollicular and luteal phase of themenstrual cycle. Pre-operativeanalgesia, if given, consisted of anon-steroidal anti-in¯ammatorydrug given 30±60 min prior tothe procedure. Women receiveda paracervical block only, or insome cases, withintravenous sedation. Generalanaesthesia was rarely used.(i) the safety of the micro-insertplacement; (ii) tolerance of andrecovery from the placementprocedure; (iii) patient experienceduring the 3 years after placement; (iv)evaluation of tubal occlusion; and (v)effectiveness at preventing pregnancy.Outcome data were measured as<strong>de</strong>scribed in Kerin 2000. Extrareported outcome = dyspareuniaAs well follow ups at 6 months, 12 and 18 months are <strong>de</strong>scribed, withsevere loss to follow up.These women have accumulated 1894 womenmonths of effectiveness(time relying on the <strong>de</strong>vice forcontraception). As of 30 June 2001 there have been no pregnanciesreported in the study. This represents aneffectiveness rate of 99.4% (95% CI 97.7% to 100%).Successful bilateral placement:88% (CI 83±92%) of women (n = 200)Correct <strong>de</strong>vice placement was confirmed in 97% of cases at 3 months.Of the 200 women with initial bilateral placement, 198 hadPatient satisfaction:The majority reported that intraprocedural pain was less than or equalto that expected, and 96% rated tolerance of the <strong>de</strong>viceplacement procedure as good to excellent.Pain: Women with bilateral and unilateral placement (n = 206): 153(76%) reported pain following the procedure. Pain resolved within 1 dayin 59% of thesen within 3 days in 88% and within 1 week in 99%.Post procedure bleeding in 171/206 women (83%). Resolved within 21day in 27%, 3 days in 64% and 1 weel in 96%.Over 24 months follow-up, 98% of study participants rated theirtolerance of the micro-insert as very good to excellent.Study groups clearly <strong>de</strong>fined: +Selectionbias*: not applicabeA<strong>de</strong>quate assesment of exposure *:+A<strong>de</strong>quate assesment of outcomes*:+Assesor of the effect a<strong>de</strong>quatelyblin<strong>de</strong>d*: -Sufficient length of follow-up*: +Selective loss to83