ReferentieType studieKenmerken(studie/patiënten)Interventie (I)Uitkomstmaten enfollow-up duurResultatenBeoor<strong>de</strong>lingkwaliteit studieKnown anatomic abnormalities possiblypreventing tubal cannulationCervical or uterine neoplasia or its precursorsUntreated acute cervicitis or history of acutepelvic in¯ammatory diseaseUnexplained abnormal uterine bleedingHistory of chronic pelvic pain, severedysmenorrhoea or severe dyspareuniaPrior Fallopian tube surgeryAny unresolved tubal, ovarian or endometrialpathologyAllergy to contrast mediaInability to comply with protocolrequirementsMost women could be discharged in an ambulatory state within 1±2 h.Adverse events:in 15/227 (7%) of the women, but none was serious.follow-up*: ? not reportedI<strong>de</strong>ntification ofconfoun<strong>de</strong>rs andcorrection in analysis: -Financial support:Financiering: Fun<strong>de</strong>d by ConceptusKerin 2004ProspectivecohortstudynotcomparativeN = 102(109enrolled, 7withdrawnthemselfes)average age was 35 years (range 23±45)Inclusion criteria:having regular, cyclical menses, seekingpermanentcontraception, aged between 21 and 40 years,weighed between 40 kg to 136 kg, had at leastone live birth, willing to accept the risk ofpregnancy occurring while relying on Essurefor prevention, were in a monogamousheterosexual relationship, willing to use eitherbarrier methods or oral contraceptive pills forthe first 3 months following micro-insertplacement, and capable of un<strong>de</strong>rstanding therisks and benefits of the study, available forall study visits.Exclusion criteria:unsure about their <strong>de</strong>sire to end their fertility,known uterine or cervical neoplasia or itsprecursors, abnormal uterine bleeding,chronic pelvic pain, pelvic inflammatorydisease, previous ectopic pregnancy, orprevious salpingectomy.Age (mean/ average/ 95% CI): 36.3 ± 3.2years (SEM)BMI (mean/ average/ 95% CI)Other rele<strong>van</strong>t <strong>de</strong>mographics…Use of a new coil catheter (CC)<strong>de</strong>livery system for micro-insertplacement. (Essure)Outcomes (primary/ secondary) andassesment of outcomes:Safety of the CC <strong>de</strong>sign of the Essure<strong>de</strong>livery catheter, evaluate theplacement effectiveness of the CC<strong>de</strong>sign for placing amicro-insert into the proximalfallopian tube lumens (HSG at 3months), and evaluate its reliance ratefor pregnancy prevention.Follow-up: HSG at 3 months (94%),559 patient months follow up forpregnancies.Pregnancies:After 6015 woman-months of exposure to intercourse, no pregnancieshave been recor<strong>de</strong>d.Apart from failure to achieve bilateral micro-insert placement in 2 of102 patients (2%), there were no unexpected procedural or earlypostprocedureadverse events.At 1-week follow-up, the si<strong>de</strong> effects reported were bleeding and pain.Light vaginal bleeding or spotting was experienced by 56 of 98 women(57%) for up to 1 week following the procedure. No postprocedurepain was reported by 68 of 98 (69%) respon<strong>de</strong>nts. Thirty women (31%)experienced some pain during the first week: 16 reported it as mild, 10as mo<strong>de</strong>rate, and 4 as severe when asked to compare it to their usualpain experienced with menses.The only complication was a micro-insert perforation through theutero-tubal area that was <strong>de</strong>tected during the 3-month postprocedureHSG.average procedure time, <strong>de</strong>fined as the time from hysteroscopicinsertion to hysteroscope removal from the uterine cavity, was 8.1 ± 3.7minutes (SEM)At 1-week follow-up, satisfaction was rated as “very satisfied” by 94 of100 women (94%) and 6 of 100 (6%) were reasonably satisfied.Study groups clearly <strong>de</strong>fined: (+)Selectionbias*: not applicableA<strong>de</strong>quate assesment of exposure *:(+)A<strong>de</strong>quate assesment of outcomes*:(+/-), for safety: +Assesor of the effect a<strong>de</strong>quatelyblin<strong>de</strong>d*: (?) not applicable(outcome safety is very clear)Sufficient length of follow-up*: +(for safety of procedureSelective loss tofollow-up*: (?): outcomepregnancies were“passively”collected.I<strong>de</strong>ntification ofconfoun<strong>de</strong>rs andcorrection in analysis: (-).Anaesthesia could have influencedthe painscores (83 womenparacervival block, 19 womenadditional IV sedation and 1converted to general.Financial support:84
ReferentieType studieKenmerken(studie/patiënten)Interventie (I)Uitkomstmaten enfollow-up duurResultatenBeoor<strong>de</strong>lingkwaliteit studieKulier, 2002Cochrane ReviewLangenveld,2008MetaanalysiscomparativeProspectivecohortstudy,published as“casereports”notcomparativeN = 149enrolled,placementwasattempted in143 patientsInclusion criteria:Exclusion criteria:Age (mean/ average/ 95% CI)BMI (mean/ average/ 95% CI)Other rele<strong>van</strong>t <strong>de</strong>mographics…Inclusion criteria:Female patients with a request for permanenttubal sterilization.Exclusion criteria: not <strong>de</strong>scribedNo <strong>de</strong>mographic data provi<strong>de</strong>dLaparoscopic sterilizationcompared to minilaparotomyIrrespective of the technique ofthe technique used forinterrupting tubal patence.(combined with culdoscopy alsoinclu<strong>de</strong>d)Essure sterilization.Anesthesia: NSAID)Outcomes (primary/ secondary) andassesment of outcomes:Operative mortality and majormorbidity (cardiac arrest, pulmonaryembolism, intestinal or vascularinjuries requiring additional surgery)Minor morbidity (intestinal or vascularinjuries not requiring additionalsurgery, post operative woundhaematoma or infectionnot requiring hospitalisation, urinarytract infection)Failure of surgical approach(laparoscopy converted intolaparotomyor extension of the mini-laparotomyincision), failure ofanaesthetic approach, duration ofoperation, hospital stay > 24hours, complaints (abdominal pain,analgesic use post-operatively,persistent abdominal pain at follow-up,other minor complaintsat follow-up)Duration of operation and length ofhospital stayWomen’s satisfaction (as questioned atfollow-up)Follow-up:Primary outcome:Bilateral tubal occlusion after Essuresterilization and complication rate.Secondary outcome:The use ofultrasound as diagnostic tool forverification of proper placement forthe 3-month follow-up.Follow-up: 3 monthsSix trials were inclu<strong>de</strong>d in the review.Minilaparotomy vs laparoscopy: There was no difference in majormorbidity between the 2 groups. Minor morbidity was significantlyless in the laparoscopy group (Peto OR 1.89; 95% CI 1.38, 2.59).Duration of operation was shorter with laparoscopy (WMD 5.34;95% CI 4.52, 6.16).Minilaparotomy vs culdoscopy: Major morbidity was higher forculdoscopy compared to minilaparotomy (Peto OR 0.14; 95% CI0.02, 0.98). Duration of operation was shorter with culdoscopy (WMD4.91; 95% CI 3.82, 6.01).Laparoscopy vs culdoscopy: In the one trial comparing the twointerventions there was no significant difference between the groupswith regard to major morbidity. Significantly more women sufferedfrom minor morbidities with culdoscopy (Peto OR 0.20; 95% CI- 0.05, 0.77).In 149 placement was attempted, in 6 patients <strong>de</strong>vice placement was notattempted (3 patients no access to the uterine cavity, 2 patients in whichthe tubal ostia could not be seen, and 1 vasovagal collapse)- Bilateral placement of the <strong>de</strong>vice was successful in(136/143) 95%(95% confi<strong>de</strong>nce interval [CI] 92%–99%). Seven attempts wereunsuccessful. The complication rate was 2% (n = 3), and all involveda perforation.Vaginal ultrasound was conclusive in 91.7% (95% CI 87%–96%) nb nocomparison with a standard was ma<strong>de</strong>.; two perforations were notrecognized on the ultrasound. In 11 patients (8.3%) extra diagnostictests were performed (X-ray or HSG). In two patients an incorrectposition due to tubal perforation was not recognized.Study groups clearly <strong>de</strong>fined: (+)Selectionbias*: (+/-):three trialsreceived a scoreof ´ B´ for unclear methods ofrandomisation and of concealmentof allocation.A<strong>de</strong>quate assesment of exposure *:(+/-): in three trials allocationconcealment was judges unclearA<strong>de</strong>quate assesment of outcomes*:(+)Assesor of the effect a<strong>de</strong>quatelyblin<strong>de</strong>d*: (-)Sufficient length of follow-up*: (+)Selective loss tofollow-up*: (-)I<strong>de</strong>ntification ofconfoun<strong>de</strong>rs andcorrection in analysis: (+)Financial support: -Study groups clearly <strong>de</strong>fined: +Selectionbias*: nvtA<strong>de</strong>quate assesment of exposure *:+A<strong>de</strong>quate assesment of outcomes*:(+)Assesor of the effect a<strong>de</strong>quatelyblin<strong>de</strong>d*: -Sufficient length of follow-up*: +Selective loss tofollow-up*: -85
- Page 5 and 6:
Conclusies ........................
- Page 7 and 8:
Onderbouwing en overwegingen ......
- Page 9 and 10:
Gezien de risico’s van een laparo
- Page 11 and 12:
Hysteroscopische sterilisaties moet
- Page 13 and 14:
Afbakening en uitgangsvragenDe huid
- Page 15 and 16:
In eerste instantie werd gezocht na
- Page 17 and 18:
Tabel 2. Niveau van bewijskracht va
- Page 19 and 20:
Implementatie en indicatorontwikkel
- Page 21 and 22:
HOOFDSTUK 1AchtergrondinformatieInl
- Page 23 and 24:
altijd werd aangenomen en dat de le
- Page 25 and 26:
Peterson, H. B., Xia, Z., Hughes, J
- Page 27 and 28:
Veiligheid2.1 Wat is meest optimale
- Page 29 and 30:
Bipolaire coagulatie versus Filshie
- Page 31 and 32:
hebben geantwoord, waarbij een succ
- Page 33 and 34: Klinische relevantie: kritiek / bel
- Page 35 and 36: Ringen 5,9 (3,3 - 8,5) 10,0 (6,4 -
- Page 37 and 38: Van alle in Nederland beschikbare s
- Page 39 and 40: LiteratuurAranda, C., Broutin, A.,
- Page 41 and 42: HOOFDSTUK 3Keuze van sterilisatieme
- Page 43 and 44: mislukken van de sterilisatie.In de
- Page 45 and 46: Uterus myomatosus en andere uterusa
- Page 47 and 48: Niveau 3En zijn aanwijzingen dat ee
- Page 49 and 50: HOOFDSTUK 4CounselingUitgangsvraag4
- Page 51 and 52: Kans op spijtDe meeste vrouwen neme
- Page 53 and 54: - Algemene voorgeschiedenis- Kans o
- Page 55 and 56: terwijl soms ook de hulp van een ma
- Page 57 and 58: een open verbinding - fistel - onts
- Page 59 and 60: Stel de zwangerschapstermijn vast e
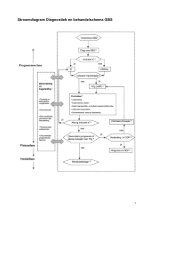
- Page 61 and 62: HOOFDSTUK 6KwaliteitscriteriaUitgan
- Page 63 and 64: InleidingAls na een sterilisatie ee
- Page 65 and 66: BevestigingstestBij alle laparoscop
- Page 67 and 68: Bij analyse van zwangerschappen na
- Page 69 and 70: BIJLAGE 1 ZoekverantwoordingDeze ri
- Page 71 and 72: ReferentieType studieKenmerken(stud
- Page 73 and 74: ReferentieType studieKenmerken(stud
- Page 75 and 76: ReferentieType studieKenmerken(stud
- Page 77 and 78: ReferentieType studieKenmerken(stud
- Page 79 and 80: ReferentieType studieKenmerken(stud
- Page 81 and 82: ReferentieType studieKenmerken(stud
- Page 83: ReferentieType studieKenmerken(stud
- Page 87 and 88: ReferentieType studieKenmerken(stud
- Page 89 and 90: ReferentieType studieKenmerken(stud
- Page 91 and 92: ReferentieType studieKenmerken(stud
- Page 93 and 94: ReferentieType studieKenmerken(stud
- Page 95 and 96: ReferentieType studieKenmerken(stud
- Page 97 and 98: ReferenceType studyCharacteristicsI
- Page 99 and 100: ReferenceType studyCharacteristicsI
- Page 101 and 102: ReferenceType studyCharacteristicsI
- Page 103 and 104: ReferenceType studyCharacteristicsI
- Page 105 and 106: ReferenceType studyCharacteristicsI
- Page 107 and 108: ReferenceType studyCharacteristicsI
- Page 109 and 110: ReferenceType studyCharacteristicsI
- Page 111 and 112: ReferenceType studyCharacteristicsI
- Page 113 and 114: ReferenceType studyCharacteristicsI
- Page 115 and 116: ReferenceType studyCharacteristicsI
- Page 117 and 118: * Uitkomst: de uitkomst en gebruikt
- Page 119 and 120: 119
- Page 121 and 122: 121