Raport de cercetare - Lorentz JÄNTSCHI
Raport de cercetare - Lorentz JÄNTSCHI
Raport de cercetare - Lorentz JÄNTSCHI
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
y' = (y1', y2', …, ym') = Xc = Hy<br />
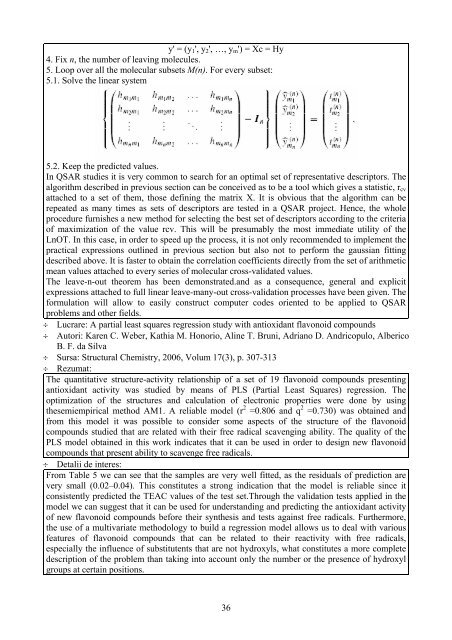
4. Fix n, the number of leaving molecules.<br />
5. Loop over all the molecular subsets M(n). For every subset:<br />
5.1. Solve the linear system<br />
5.2. Keep the predicted values.<br />
In QSAR studies it is very common to search for an optimal set of representative <strong>de</strong>scriptors. The<br />
algorithm <strong>de</strong>scribed in previous section can be conceived as to be a tool which gives a statistic, rcv<br />
attached to a set of them, those <strong>de</strong>fining the matrix X. It is obvious that the algorithm can be<br />
repeated as many times as sets of <strong>de</strong>scriptors are tested in a QSAR project. Hence, the whole<br />
procedure furnishes a new method for selecting the best set of <strong>de</strong>scriptors according to the criteria<br />
of maximization of the value rcv. This will be presumably the most immediate utility of the<br />
LnOT. In this case, in or<strong>de</strong>r to speed up the process, it is not only recommen<strong>de</strong>d to implement the<br />
practical expressions outlined in previous section but also not to perform the gaussian fitting<br />
<strong>de</strong>scribed above. It is faster to obtain the correlation coefficients directly from the set of arithmetic<br />
mean values attached to every series of molecular cross-validated values.<br />
The leave-n-out theorem has been <strong>de</strong>monstrated.and as a consequence, general and explicit<br />
expressions attached to full linear leave-many-out cross-validation processes have been given. The<br />
formulation will allow to easily construct computer co<strong>de</strong>s oriented to be applied to QSAR<br />
problems and other fields.<br />
÷ Lucrare: A partial least squares regression study with antioxidant flavonoid compounds<br />
÷ Autori: Karen C. Weber, Kathia M. Honorio, Aline T. Bruni, Adriano D. Andricopulo, Alberico<br />
B. F. da Silva<br />
÷ Sursa: Structural Chemistry, 2006, Volum 17(3), p. 307-313<br />
÷ Rezumat:<br />
The quantitative structure-activity relationship of a set of 19 flavonoid compounds presenting<br />
antioxidant activity was studied by means of PLS (Partial Least Squares) regression. The<br />
optimization of the structures and calculation of electronic properties were done by using<br />
thesemiempirical method AM1. A reliable mo<strong>de</strong>l (r 2 =0.806 and q 2 =0.730) was obtained and<br />
from this mo<strong>de</strong>l it was possible to consi<strong>de</strong>r some aspects of the structure of the flavonoid<br />
compounds studied that are related with their free radical scavenging ability. The quality of the<br />
PLS mo<strong>de</strong>l obtained in this work indicates that it can be used in or<strong>de</strong>r to <strong>de</strong>sign new flavonoid<br />
compounds that present ability to scavenge free radicals.<br />
÷ Detalii <strong>de</strong> interes:<br />
From Table 5 we can see that the samples are very well fitted, as the residuals of prediction are<br />
very small (0.02–0.04). This constitutes a strong indication that the mo<strong>de</strong>l is reliable since it<br />
consistently predicted the TEAC values of the test set.Through the validation tests applied in the<br />
mo<strong>de</strong>l we can suggest that it can be used for un<strong>de</strong>rstanding and predicting the antioxidant activity<br />
of new flavonoid compounds before their synthesis and tests against free radicals. Furthermore,<br />
the use of a multivariate methodology to build a regression mo<strong>de</strong>l allows us to <strong>de</strong>al with various<br />
features of flavonoid compounds that can be related to their reactivity with free radicals,<br />
especially the influence of substitutents that are not hydroxyls, what constitutes a more complete<br />
<strong>de</strong>scription of the problem than taking into account only the number or the presence of hydroxyl<br />
groups at certain positions.<br />
36