Thesis Title: Subtitle - NMR Spectroscopy Research Group
Thesis Title: Subtitle - NMR Spectroscopy Research Group
Thesis Title: Subtitle - NMR Spectroscopy Research Group
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
5.1 The use of PCS for structure determination. 131<br />
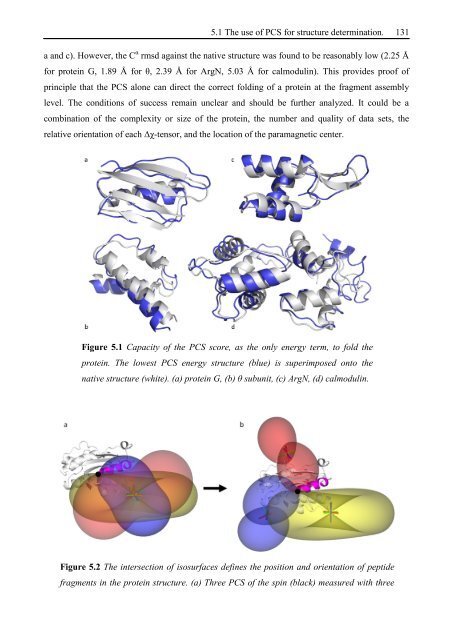
a and c). However, the C α rmsd against the native structure was found to be reasonably low (2.25 Å<br />
for protein G, 1.89 Å for θ, 2.39 Å for ArgN, 5.03 Å for calmodulin). This provides proof of<br />
principle that the PCS alone can direct the correct folding of a protein at the fragment assembly<br />
level. The conditions of success remain unclear and should be further analyzed. It could be a<br />
combination of the complexity or size of the protein, the number and quality of data sets, the<br />
relative orientation of each Δχ-tensor, and the location of the paramagnetic center.<br />
Figure 5.1 Capacity of the PCS score, as the only energy term, to fold the<br />
protein. The lowest PCS energy structure (blue) is superimposed onto the<br />
native structure (white). (a) protein G, (b) θ subunit, (c) ArgN, (d) calmodulin.<br />
Figure 5.2 The intersection of isosurfaces defines the position and orientation of peptide<br />
fragments in the protein structure. (a) Three PCS of the spin (black) measured with three