Thesis Title: Subtitle - NMR Spectroscopy Research Group
Thesis Title: Subtitle - NMR Spectroscopy Research Group
Thesis Title: Subtitle - NMR Spectroscopy Research Group
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
2.4 Results. 47<br />
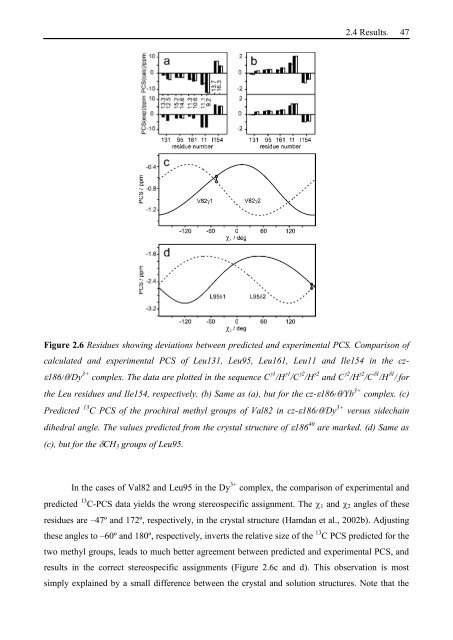
Figure 2.6 Residues showing deviations between predicted and experimental PCS. Comparison of<br />
calculated and experimental PCS of Leu131, Leu95, Leu161, Leu11 and Ile154 in the cz-<br />
186/ /Dy 3+ complex. The data are plotted in the sequence C 1 /H 1 /C 2 /H 2 and C 2 /H 2 /C 1 /H 1 / for<br />
the Leu residues and Ile154, respectively. (b) Same as (a), but for the cz- 186/ /Yb 3+ complex. (c)<br />
Predicted 13 C PCS of the prochiral methyl groups of Val82 in cz- 186/ /Dy 3+ versus sidechain<br />
dihedral angle. The values predicted from the crystal structure of 186 40 are marked. (d) Same as<br />
(c), but for the CH3 groups of Leu95.<br />
In the cases of Val82 and Leu95 in the Dy 3+ complex, the comparison of experimental and<br />
predicted 13 C-PCS data yields the wrong stereospecific assignment. The 1 and 2 angles of these<br />
residues are –47º and 172º, respectively, in the crystal structure (Hamdan et al., 2002b). Adjusting<br />
these angles to –60º and 180º, respectively, inverts the relative size of the 13 C PCS predicted for the<br />
two methyl groups, leads to much better agreement between predicted and experimental PCS, and<br />
results in the correct stereospecific assignments (Figure 2.6c and d). This observation is most<br />
simply explained by a small difference between the crystal and solution structures. Note that the