- Page 1: ./0123/M./T!6 1MP!8T ST!T.M./T 1a$t
- Page 4 and 5: Project director David Gwyther Proj
- Page 7: Appendix 8 Juvenile Amphipod Whole
- Page 10 and 11: 10-day Survival of M. plumulosa Pag
- Page 12 and 13: Centre for Environmental Contaminan
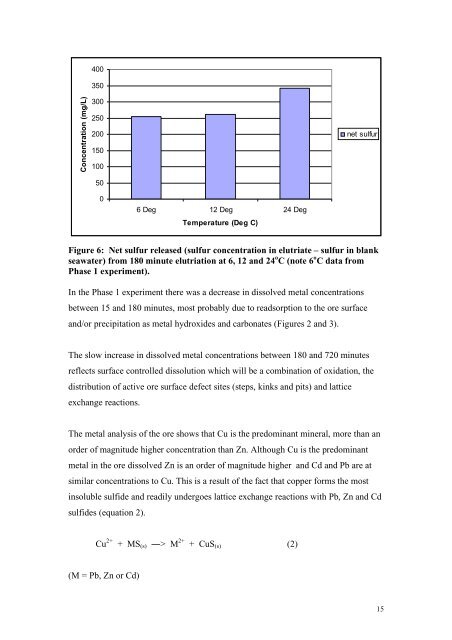
- Page 14 and 15: Centre for Environmental Contaminan
- Page 17: Appendix 9 Elutriate Testing Report
- Page 20 and 21: Table of Contents 1. Introduction 3
- Page 22 and 23: These elutriate tests were designed
- Page 24 and 25: 2. An aliquot(s) of sample after ce
- Page 26 and 27: Table 1: pH, redox (Eh), conductivi
- Page 28 and 29: 1440 minutes which is also consiste
- Page 30 and 31: Concentration (ppb) 250.0 200.0 150
- Page 34 and 35: Zinc sulfide is the most soluble of
- Page 36 and 37: Table 5: Major ions in 0.45 !m filt
- Page 38 and 39: 3.5 Comparison to ANZECC/ARMCANZ (2
- Page 40: 5. References ANZECC/ARMCANZ (2000)
- Page 43 and 44: Appendix 2: Phase 1: Metal concentr
- Page 45 and 46: Appendix 4: Phase 1: Normalised dat
- Page 47 and 48: Appendix 6: Phase 2: Quality contro
- Page 49: Appendix 8: Metal concentrations in
- Page 53 and 54: ! ! ! ! ! ! !"#$%!"#$#%&! ! !
- Page 55 and 56: ! ! ! ! ! ! !"#$%!&''(! ! !"#$%!"#$
- Page 57 and 58: ! !"#$%!&''(! ! !"#$%!"#$#%&'()&'*)
- Page 59 and 60: ! ! !"#$%!"#$#%&'()&'*)+ 7&89:
- Page 61 and 62: ! !"#$%!&''(! ! !"#$%!"#$#%&'()&'*)
- Page 63 and 64: ! !"#$%!&''(! ! !"#$%!"#$#%&'()&'
- Page 65 and 66: ! ! !"#$%!&''(! !
- Page 67 and 68: ! !"#$%!&''(! ! !"#$%!"#$#%&'()&'*)
- Page 69 and 70: ! !"#$%!&''(! ! !"#$%!"#$#%&'()&'*)
- Page 71 and 72: ! !"#$%!&''(! ! !"#$%!"#$#%&'()&'*)
- Page 73 and 74: ! !"#$%!&''(! ! !"#$%!"#$#%&'()&'*)
- Page 75 and 76: ! 3-8'!C&:#!%?$6:$%!-G!98#&!&'$!I#-
- Page 77 and 78: ! !"#$%!&''(! ! !"#$%!"#$#%&'()&'*)
- Page 79 and 80: ! !"#$%!&''(! ! !"#$%!"#$#%&'()&'*)
- Page 81 and 82: !"#$%!"#$#%&'()&'*)+ ! ! &O !"#$
- Page 83 and 84:
! !"#$%!&''(! ! !"#$%!"#$#%&'()&'*)
- Page 85 and 86:
!"#$%!"#$#%&'()&'*)+ ! ! ! &, !"#
- Page 87 and 88:
! !"#$%!&''(! ! !"#$%!"#$#%&'()&'*)
- Page 89 and 90:
! !"#$%!&''(! ! !"#$%!"#$#%&'()&
- Page 91 and 92:
! !"#$%!&''(! ! !"#$%!"#$#%&'()
- Page 93 and 94:
! !"#$%!&''(! ! !"#$%!"#$#%&'()&'*)
- Page 95 and 96:
! !"#$%!&''(! ! ! ! ! ! !"#$%
- Page 97 and 98:
! ! ! !"#$%!&''(! ! !"#$%!"#$
- Page 99 and 100:
! !"#$%!&''(! ! !"#$%!"#$#%&'()
- Page 101 and 102:
! 7&.&#-:5!6-?$?-5!B81$#:.$%! 7
- Page 103 and 104:
!"#$%!"#$#%&'()&'*)+ ! ! ! ! ! ! !
- Page 105 and 106:
!"#$%!"#$#%&'()&'*)+ ! ! ! !
- Page 107 and 108:
! ! !"#$%!"#$#%&'()&'*)+ ,&C?.:#
- Page 109 and 110:
! !"#$%!&''(! ! !"#$%!"#$#%&'()&'*)
- Page 111 and 112:
! !"#$%!&''(! ! !"#$%!"#$#%&'()&'*)
- Page 113 and 114:
! !"#$%!&''(! ! !"#$%!"#$#%&'()&'*)
- Page 115 and 116:
! !"#$%!&''(! ! !"#$%!"#$#%&'()&'
- Page 117 and 118:
!"#$%!"#$#%&'()&'*)+ ! !"#$%!&''(!
- Page 119 and 120:
!"#$%!"#$#%&'()&'*)+ ! !"#$%!&''(!
- Page 121 and 122:
!"#$%!"#$#%&'()&'*)+ ! ! ! ! !"#$
- Page 123 and 124:
! !"#$%!&''(! ! !"#$%!"#$#%&'()&'*)
- Page 125 and 126:
! !"#$%!&''(! ! !"#$%!"#$#%&'()&'*)
- Page 127 and 128:
!"#$%!"#$#%&'()&'*)+ ! ! ! ! !"#$
- Page 129 and 130:
! !"#$%!&''(! ! !"#$%!"#$#%&'()&'*)
- Page 131 and 132:
!"#$%!"#$#%&'()&'*)+ ! ! 251
- Page 133 and 134:
!"#$%!"#$#%&'()&'*)+ ! Z-/! ! ]$'M
- Page 135 and 136:
! !"#$%!&''(! ! !"#$%!"#$#%&'()&'
- Page 137 and 138:
! !"#$%!&''(! ! !"#$%!"#$#%&'()&'*)
- Page 139 and 140:
! !"#$%!&''(! ! !"#$%!"#$#%&'()&'*)
- Page 141 and 142:
! !"#$%!&''(! ! !"#$%!"#$#%&'()&'*)
- Page 143 and 144:
! ! !"#$%!&'',! !"#$%!"#$#%&'()&'*)
- Page 145 and 146:
! ! !! ! ! ! 4#7:84C4760#7 IC#%!240
- Page 147 and 148:
! !!37%#;134CC6;4 >C%$;%8! J867:C%2
- Page 149 and 150:
! !"#$%!&''(! ! !"#$%!"#$#%&'()&'*)
- Page 151 and 152:
! !"#$%!&''(! ! !"#$%!"#$#%&'()&'*)
- Page 153:
! !"#$%!&''(! ! !"#$%!"#$#%&'()&'*
- Page 157 and 158:
MODELLING THE DISPERSION AND SETTLE
- Page 159 and 160:
TABLE OF CONTENTS 1 EXECUTIVE SUMMA
- Page 161 and 162:
2 MODELLING METHODOLOGY AND DATA 2.
- Page 163 and 164:
2.3 The SSFATE Model Sediment dispe
- Page 165 and 166:
Earth Mechanics Institute of the Co
- Page 167 and 168:
3 RESULTS AND DISCUSSION The calcul
- Page 169 and 170:
Table 3.1. Summary of areas covered
- Page 171 and 172:
Figure 3.3b. Sediment bottom thickn
- Page 173 and 174:
Figure 3.3d. Sediment bottom thickn
- Page 175:
4 REFERENCES Swanson J.C., Isaji T.
- Page 179 and 180:
MODELLING THE DISPERSION OF THE RET
- Page 181 and 182:
TABLE OF CONTENTS 1 EXECUTIVE SUMMA
- Page 183 and 184:
Given the range of tidal and basin
- Page 185 and 186:
dispersion is modelled using a rand
- Page 187 and 188:
Figure 2.1. Data from the ADCP at 6
- Page 189 and 190:
2.5 Outfall Configuration and Assum
- Page 191 and 192:
3 RESULTS AND DISCUSSION 3.1 Diluti
- Page 193 and 194:
Figure 3.1. Snapshot in time of a
- Page 195 and 196:
5.0 km at any time during the disch
- Page 197 and 198:
Figure 3.2. The expected (or averag
- Page 199 and 200:
Figure 3.4 A zoomed in comparison o
- Page 201 and 202:
5 REFERENCES Bear, J. and Verruijt,
- Page 203:
Rusin J., Lunel T. and Davies L., 1
- Page 207 and 208:
Prediction of underwater noise asso
- Page 209 and 210:
Table of Contents 1 Introduction ..
- Page 211 and 212:
1 Introduction This report presents
- Page 213 and 214:
2 Methods 2.1! Source modelling Cav
- Page 215 and 216:
dB re 1uPa @ 1m 200 195 190 185 180
- Page 217 and 218:
Figure 3. Source location for the s
- Page 219 and 220:
Depth (m) 0 500 1000 1500 2000 2500
- Page 221 and 222:
Figure 7. Plots of predicted receiv
- Page 223 and 224:
Figure 9. Zoomed view of plots of p
- Page 225 and 226:
Figure 11. Predicted maximum level
- Page 227 and 228:
for computing a is given in Fisher
- Page 229 and 230:
ecent studies have indicated that c
- Page 231 and 232:
sources to close range, and that th
- Page 233 and 234:
masking ranges for different marina
- Page 235 and 236:
References Fisher, F. H., Simmons,
- Page 237:
Appendix 14 The Potential for Natur
- Page 240 and 241:
'leaky' and recent lava flows have
- Page 242 and 243:
iii.) Incrementally these 1 m swath
- Page 247:
Appendix 15 Stakeholder Consultatio
- Page 250 and 251:
Environmental Impact Statement Solw
- Page 252 and 253:
Environmental Impact Statement Solw
- Page 254 and 255:
Environmental Impact Statement Solw
- Page 256 and 257:
Wewak (22 October 2007) Not recorde
- Page 258:
San Diego (17-18 April 2008) Enviro