impaginato piccolo - Società Italiana di Parassitologia (SoIPa)
impaginato piccolo - Società Italiana di Parassitologia (SoIPa)
impaginato piccolo - Società Italiana di Parassitologia (SoIPa)
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
12<br />
therefore of the Th1 development (Farrar 2002).<br />
Another transcription factor that is specific for Th2<br />
cells is c-MAF, which is also responsible for regulating<br />
IL-4 synthesis through the activation of the IL-4 promoter<br />
( Ho 1996). Once GATA-3 production reaches a<br />
certain threshold, its own gene expression is auto-activated,<br />
hence stabilizing the Th2 phenotype through an<br />
intrinsic positive-feedback loop (Farrar 2002). As Th2<br />
cells mature, they produce increasing levels of IL-4,<br />
which generates a paracrine loop and induces neighboring<br />
naive T cells to develop into Th2 cells.<br />
IL-6, another cytokine released by macrophages, mast<br />
cells and pulmonary DCs during the early stages of a<br />
Th2 response, induces the Th2 phenotype through the<br />
up-regulation of IL-4 and inhibition of STAT1 phosphorylation,<br />
thereby preventing IFN-γ gene expression<br />
(Dodge 2003, Detournay 2005). In humans, IL-11<br />
released by myeloid cells acts <strong>di</strong>rectly on T cells to stimulate<br />
IL-4 and IL-5 expression, while simultaneously<br />
inhibits IFN-γ production. IL-11 also suppresses IL-12<br />
secretion and therefore also contributes to Th2 <strong>di</strong>fferentiation<br />
through this in<strong>di</strong>rect mechanism ( Curti<br />
2001). Activated DC2 may induce Th2 <strong>di</strong>fferentiation<br />
in<strong>di</strong>rectly via the secretion of IL-10, which then inhibits<br />
IL-12 synthesis at mRNA level and thus the Th1 pathway<br />
(Koch 1996). IL-10 also down-regulates IL-12β2R<br />
expression (Romano 2005), suggesting that the development<br />
of the Th2 phenotype would be the default<br />
pathway, occurring spontaneously in the absence of IL-<br />
12, but this is a point of controversy (Langenkamp<br />
2000, Maldonado-López 2001). Whether DC2 secrete<br />
other soluble factors that promote Th2 cell development,<br />
remains unknown. Mast cell degranulation and<br />
me<strong>di</strong>ator release can reduce the capacity of DCs to<br />
induce Th1 cells and promote the development of IL-4secreting<br />
T cells (Mazzoni 2006) suggesting that mast<br />
cells may have a role in the development of the antigenspecific<br />
Th2 cells in mast cell-related <strong>di</strong>sorders, such as<br />
atopy. Several coreceptors are implicated in the activation<br />
and the reinforcement of the Th2 phenotype.<br />
Following Th cell activation, the costimulatory molecule<br />
ICOS is up-regulated and is retained on both effector<br />
and memory cells (Hutloff 1999). Its ligand (ICOS-<br />
L), is expressed on most APCs, inclu<strong>di</strong>ng DCs, B cells,<br />
activated monocytes, fibroblasts and endothelial cells<br />
(Wassink 2004). ICOS participates in the regulation of<br />
T-cell activation by supporting the release of many<br />
cytokines (Okamoto 2004). ICOS –/– T cells are selectively<br />
deficient in IL-4 production (Nurieva 2005), and<br />
inhibition of ICOS activity results in the arrest of Th2<br />
cell-me<strong>di</strong>ated allergic airway responses without change<br />
of Th1-me<strong>di</strong>ated IFN-γ secretion (Coyle 2000).<br />
Regulation of Th1/Th2 responses<br />
An impressive series of in vitro and in vivo data<br />
obtained in both experimental animals and humans,<br />
have shown that Th1 and Th2 responses are mutually<br />
regulated in a process known as re-<strong>di</strong>rection or immune<br />
deviation. IL-12, IL-18, IFN-γ and IFN-α not only<br />
G. Del Prete - The complexity of the CD4 T-cell response<br />
favour the development of Th1 cells, but also inhibit<br />
the development of Th2 cells. Likewise, a number of<br />
pathogen products or even synthetic adjuvants, which<br />
are agonists of TLRs present on DCs and/or NK cells<br />
and are able to induce the production of IL-12 and/or<br />
IFNs by cells of the innate immunity, promote the shifting<br />
of Th2 responses to the less polarized Th0, or even<br />
to the Th1 polarized profile and effector functions (Erb<br />
1998, Klinman 2004, Mohamadzadeh 2005, Revets<br />
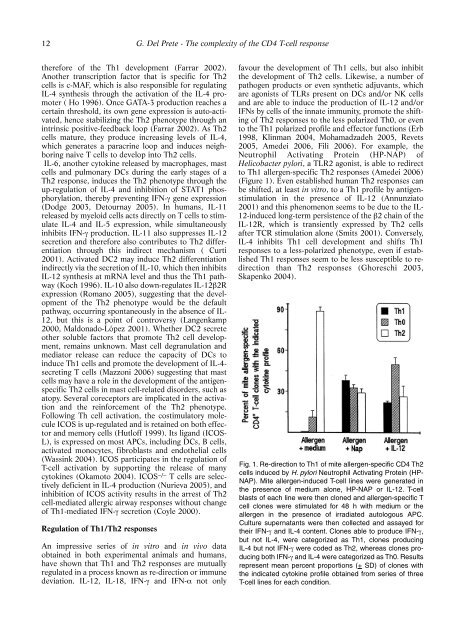
2005, Amedei 2006, Filì 2006). For example, the<br />
Neutrophil Activating Protein (HP-NAP) of<br />
Helicobacter pylori, a TLR2 agonist, is able to re<strong>di</strong>rect<br />
to Th1 allergen-specific Th2 responses (Amedei 2006)<br />
(Figure 1). Even established human Th2 responses can<br />
be shifted, at least in vitro, to a Th1 profile by antigenstimulation<br />
in the presence of IL-12 (Annunziato<br />
2001) and this phenomenon seems to be due to the IL-<br />
12-induced long-term persistence of the β2 chain of the<br />
IL-12R, which is transiently expressed by Th2 cells<br />
after TCR stimulation alone (Smits 2001). Conversely,<br />
IL-4 inhibits Th1 cell development and shifts Th1<br />
responses to a less-polarized phenotype, even if established<br />
Th1 responses seem to be less susceptible to re<strong>di</strong>rection<br />
than Th2 responses (Ghoreschi 2003,<br />
Skapenko 2004).<br />
Fig. 1. Re-<strong>di</strong>rection to Th1 of mite allergen-specific CD4 Th2<br />
cells induced by H. pylori Neutrophil Activating Protein (HP-<br />
NAP). Mite allergen-induced T-cell lines were generated in<br />
the presence of me<strong>di</strong>um alone, HP-NAP or IL-12. T-cell<br />
blasts of each line were then cloned and allergen-specific T<br />
cell clones were stimulated for 48 h with me<strong>di</strong>um or the<br />
allergen in the presence of irra<strong>di</strong>ated autologous APC.<br />
Culture supernatants were then collected and assayed for<br />
their IFN-γ and IL-4 content. Clones able to produce IFN-γ,<br />
but not IL-4, were categorized as Th1, clones producing<br />
IL-4 but not IFN-γ were coded as Th2, whereas clones producing<br />
both IFN-γ and IL-4 were categorized as Th0. Results<br />
represent mean percent proportions (+ SD) of clones with<br />
the in<strong>di</strong>cated cytokine profile obtained from series of three<br />
T-cell lines for each con<strong>di</strong>tion.