impaginato piccolo - Società Italiana di Parassitologia (SoIPa)
impaginato piccolo - Società Italiana di Parassitologia (SoIPa)
impaginato piccolo - Società Italiana di Parassitologia (SoIPa)
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
28<br />
affected by malaria cannot be overstated or ignored.<br />
The WHO even published its guidelines in December<br />
of 2005, for the first time stating that artemisinins are<br />
first line therapy for much of malaria. These recommendations<br />
<strong>di</strong>d not take long to catch on in most of the<br />
world most severely affected by malaria. Table 1 shows<br />
the governmentally accepted first line artemisinin therapy<br />
recommendations as of 2007.<br />
Team,” there was a momentous decision to be made,<br />
and that was that quini<strong>di</strong>ne must be replaced. To<br />
understand the importance of this decision, one must<br />
first understand a little about quini<strong>di</strong>ne. While this<br />
drug has saved the lives of people with severe malaria<br />
in the United States over the time it has been available,<br />
it has toxicities that require admission to an intensive<br />
care unit to safely give the drug. Like its cousin quinine,<br />
quini<strong>di</strong>ne has several side effects associated with<br />
its use. Quini<strong>di</strong>ne happens to be “better” for the treatment<br />
of malaria, as far as how rapidly it works, but it<br />
is also much more toxic than its cousin quinine. Among<br />
the numerous side effects common to both drugs is<br />
prolongation of the “Q-T” interval. This action of the<br />
drug is why quini<strong>di</strong>ne was originally licensed in the<br />
United States. It was a pro-arrhythmic drug used to<br />
cause changes in the heart’s electrical impulses and correct<br />
unstable electrical rhythms in car<strong>di</strong>ology patients.<br />
The fact that quini<strong>di</strong>ne was already licensed for car<strong>di</strong>ac<br />
reasons in the U.S., and the fact that it was more effective<br />
for treating malaria than even quinine, made it a<br />
perfect can<strong>di</strong>date for being the drug of choice for<br />
severe malaria. But this ease in licensing came at a<br />
price, and this price was more <strong>di</strong>fficult and costly treatment<br />
of severe malaria in intensive care units.<br />
P. J. Weina - History of Artemisinins<br />
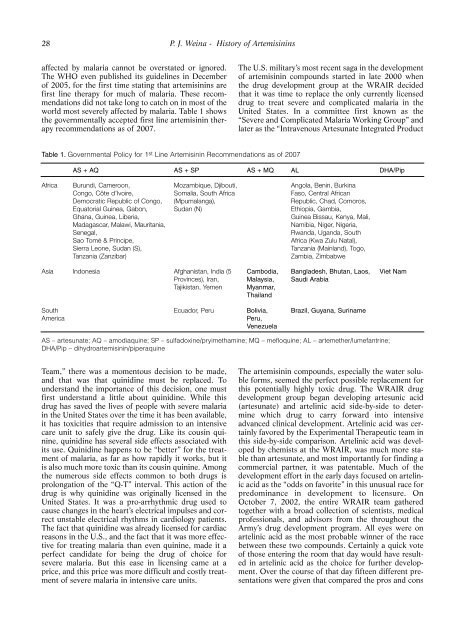
Table 1. Governmental Policy for 1 st Line Artemisinin Recommendations as of 2007<br />
The U.S. military’s most recent saga in the development<br />
of artemisinin compounds started in late 2000 when<br />
the drug development group at the WRAIR decided<br />
that it was time to replace the only currently licensed<br />
drug to treat severe and complicated malaria in the<br />
United States. In a committee first known as the<br />
“Severe and Complicated Malaria Working Group” and<br />
later as the “Intravenous Artesunate Integrated Product<br />
AS + AQ AS + SP AS + MQ AL DHA/Pip<br />
Africa Burun<strong>di</strong>, Cameroon,<br />
Congo, Côte d’Ivoire,<br />
Democratic Republic of Congo,<br />
Equatorial Guinea, Gabon,<br />
Ghana, Guinea, Liberia,<br />
Madagascar, Malawi, Mauritania,<br />
Senegal,<br />
Sao Tomé & Principe,<br />
Sierra Leone, Sudan (S),<br />
Tanzania (Zanzibar)<br />
Mozambique, Djibouti,<br />
Somalia, South Africa<br />
(Mpumalanga),<br />
Sudan (N)<br />
Asia Indonesia Afghanistan, In<strong>di</strong>a (5<br />
Provinces), Iran,<br />
Tajikistan, Yemen<br />
South<br />
America<br />
Cambo<strong>di</strong>a,<br />
Malaysia,<br />
Myanmar,<br />
Thailand<br />
Ecuador, Peru Bolivia,<br />
Peru,<br />
Venezuela<br />
Angola, Benin, Burkina<br />
Faso, Central African<br />
Republic, Chad, Comoros,<br />
Ethiopia, Gambia,<br />
Guinea Bissau, Kenya, Mali,<br />
Namibia, Niger, Nigeria,<br />
Rwanda, Uganda, South<br />
Africa (Kwa Zulu Natal),<br />
Tanzania (Mainland), Togo,<br />
Zambia, Zimbabwe<br />
Bangladesh, Bhutan, Laos,<br />
Sau<strong>di</strong> Arabia<br />
Brazil, Guyana, Suriname<br />
AS – artesunate; AQ – amo<strong>di</strong>aquine; SP – sulfadoxine/pryimethamine; MQ – mefloquine; AL – artemether/lumefantrine;<br />
DHA/Pip – <strong>di</strong>hydroartemisinin/piperaquine<br />
Viet Nam<br />
The artemisinin compounds, especially the water soluble<br />
forms, seemed the perfect possible replacement for<br />
this potentially highly toxic drug. The WRAIR drug<br />
development group began developing artesunic acid<br />
(artesunate) and artelinic acid side-by-side to determine<br />
which drug to carry forward into intensive<br />
advanced clinical development. Artelinic acid was certainly<br />
favored by the Experimental Therapeutic team in<br />
this side-by-side comparison. Artelinic acid was developed<br />
by chemists at the WRAIR, was much more stable<br />
than artesunate, and most importantly for fin<strong>di</strong>ng a<br />
commercial partner, it was patentable. Much of the<br />
development effort in the early days focused on artelinic<br />
acid as the “odds on favorite” in this unusual race for<br />
predominance in development to licensure. On<br />
October 7, 2002, the entire WRAIR team gathered<br />
together with a broad collection of scientists, me<strong>di</strong>cal<br />
professionals, and advisors from the throughout the<br />
Army’s drug development program. All eyes were on<br />
artelinic acid as the most probable winner of the race<br />
between these two compounds. Certainly a quick vote<br />
of those entering the room that day would have resulted<br />
in artelinic acid as the choice for further development.<br />
Over the course of that day fifteen <strong>di</strong>fferent presentations<br />
were given that compared the pros and cons