impaginato piccolo - Società Italiana di Parassitologia (SoIPa)
impaginato piccolo - Società Italiana di Parassitologia (SoIPa)
impaginato piccolo - Società Italiana di Parassitologia (SoIPa)
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
14<br />
mechanisms of action remain undefined (Batten 2006,<br />
Kleinschek 2007). More recently, a functional antagonism<br />
between Th17 and Foxp3 + Treg cells has been<br />
reported (Bettelli 2006). Th17 seems to originate not<br />
only as a consequence of the production of IL-23 by<br />
DCs, but mainly because of the combined activity of<br />
IL-6 and TGF-β. Since TGF-β is also involved in the<br />
generation of Treg cells (Chen 2003, Walker 2003,<br />
Vieira 2004, Allan 2005), the fact that IL-6 inhibits<br />
their development suggests the existence of a <strong>di</strong>chotomy<br />
in the generation of pathogenic Th17 cells that can<br />
induce autoimmunity and that of Foxp3 + Treg cells that<br />
inhibit autoimmunity. Intrestingly, both IL-4 and IFN-γ<br />
inhibit the development of Th17 cells (Iwakura 2006)<br />
(Figure 2), whereas it is still unclear whether Th17 cells<br />
exert any inhibitory effect on the development of Th1<br />
and Th2 cells. Likewise, the precise effects of Tregs on<br />
Th17 cells are as yet unknown.<br />
In conclusion, the development of a polarized Th cell<br />
from a naïve T-cell is a complex process involving stimulation<br />
of TLRs, and activation and maturation of DCs,<br />
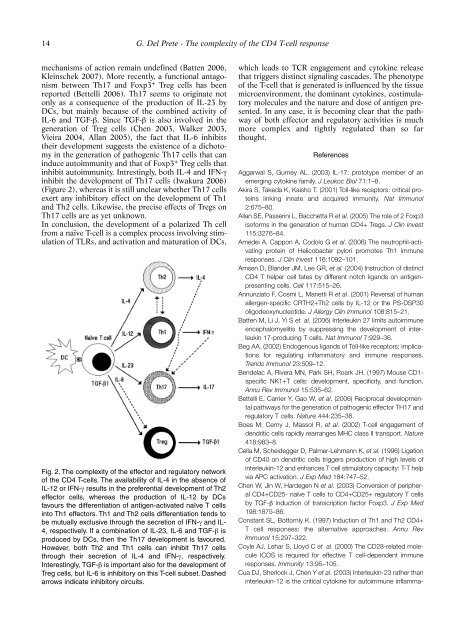
Fig. 2. The complexity of the effector and regulatory network<br />
of the CD4 T-cells. The availability of IL-4 in the absence of<br />
IL-12 or IFN-γ results in the preferential development of Th2<br />
effector cells, whereas the production of IL-12 by DCs<br />
favours the <strong>di</strong>fferentiation of antigen-activated naïve T cells<br />
into Th1 effectors. Th1 and Th2 cells <strong>di</strong>fferentiation tends to<br />
be mutually exclusive through the secretion of IFN-γ and IL-<br />
4, respectively. If a combination of IL-23, IL-6 and TGF-β is<br />
produced by DCs, then the Th17 development is favoured.<br />
However, both Th2 and Th1 cells can inhibit Th17 cells<br />
through their secretion of IL-4 and IFN-γ, respectively.<br />
Interestingly, TGF-β is important also for the development of<br />
Treg cells, but IL-6 is inhibitory on this T-cell subset. Dashed<br />
arrows in<strong>di</strong>cate inhibitory circuits.<br />
G. Del Prete - The complexity of the CD4 T-cell response<br />
which leads to TCR engagement and cytokine release<br />
that triggers <strong>di</strong>stinct signaling cascades. The phenotype<br />
of the T-cell that is generated is influenced by the tissue<br />
microenvironment, the dominant cytokines, costimulatory<br />
molecules and the nature and dose of antigen presented.<br />
In any case, it is becoming clear that the pathway<br />
of both effector and regulatory activities is much<br />
more complex and tightly regulated than so far<br />
thought.<br />
References<br />
Aggarwal S, Gurney AL. (2003) IL-17: prototype member of an<br />
emerging cytokine family. J Leukoc Biol 71:1–8.<br />
Akira S, Takeda K, Kaisho T. (2001) Toll-like receptors: critical proteins<br />
linking innate and acquired immunity. Nat Immunol<br />
2:675–80.<br />
Allan SE, Passerini L, Bacchetta R et al. (2005) The role of 2 Foxp3<br />
isoforms in the generation of human CD4+ Tregs. J Clin Invest<br />
115:3276–84.<br />
Amedei A, Cappon A, Codolo G et al. (2006) The neutrophil-activating<br />
protein of Helicobacter pylori promotes Th1 immune<br />
responses. J Clin Invest 116:1092–101.<br />
Amsen D, Blander JM, Lee GR, et al. (2004) Instruction of <strong>di</strong>stinct<br />
CD4 T helper cell fates by <strong>di</strong>fferent notch ligands on antigenpresenting<br />
cells. Cell 117:515–26.<br />
Annunziato F, Cosmi L, Manetti R et al. (2001) Reversal of human<br />
allergen-specific CRTH2+Th2 cells by IL-12 or the PS-DSP30<br />
oligodeoxynucleotide. J Allergy Clin Immunol 108:815–21.<br />
Batten M, Li J, Yi S et al. (2006) Interleukin 27 limits autoimmune<br />
encephalomyelitis by suppressing the development of interleukin<br />
17-producing T cells. Nat Immunol 7:929–36.<br />
Beg AA. (2002) Endogenous ligands of Toll-like receptors: implications<br />
for regulating inflammatory and immune responses.<br />
Trends Immunol 23:509–12.<br />
Bendelac A, Rivera MN, Park SH, Roark JH. (1997) Mouse CD1specific<br />
NK1+T cells: development, specificity, and function.<br />
Annu Rev Immunol 15:535–62.<br />
Bettelli E, Carrier Y, Gao W, et al. (2006) Reciprocal developmental<br />
pathways for the generation of pathogenic effector TH17 and<br />
regulatory T cells. Nature 444:235–38.<br />
Boes M, Cerny J, Massol R, et al. (2002) T-cell engagement of<br />
dendritic cells rapidly rearranges MHC class II transport. Nature<br />
418:983–8.<br />
Cella M, Scheidegger D, Palmer-Lehmann K, et al. (1996) Ligation<br />
of CD40 on dendritic cells triggers production of high levels of<br />
interleukin-12 and enhances T cell stimulatory capacity: T-T help<br />
via APC activation. J Exp Med 184:747–52.<br />
Chen W, Jin W, Hardegen N et al. (2003) Conversion of peripheral<br />
CD4+CD25- naïve T cells to CD4+CD25+ regulatory T cells<br />
by TGF-β induction of transcription factor Foxp3. J Exp Med<br />
198:1875–86.<br />
Constant SL, Bottomly K. (1997) Induction of Th1 and Th2 CD4+<br />
T cell responses: the alternative approaches. Annu Rev<br />
Immunol 15:297–322.<br />
Coyle AJ, Lehar S, Lloyd C et al. (2000) The CD28-related molecule<br />
ICOS is required for effective T cell-dependent immune<br />
responses. Immunity 13:95–105.<br />
Cua DJ, Sherlock J, Chen Y et al. (2003) Interleukin-23 rather than<br />
interleukin-12 is the critical cytokine for autoimmune inflamma-