A Feasibility Study - Aaltodoc - Aalto-yliopisto
A Feasibility Study - Aaltodoc - Aalto-yliopisto
A Feasibility Study - Aaltodoc - Aalto-yliopisto
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
early 1970s (Buros 2000). This also represents the ever increasing interest in<br />
desalination, with strong and continuously expanding markets (El-Ghonemy 2012).<br />
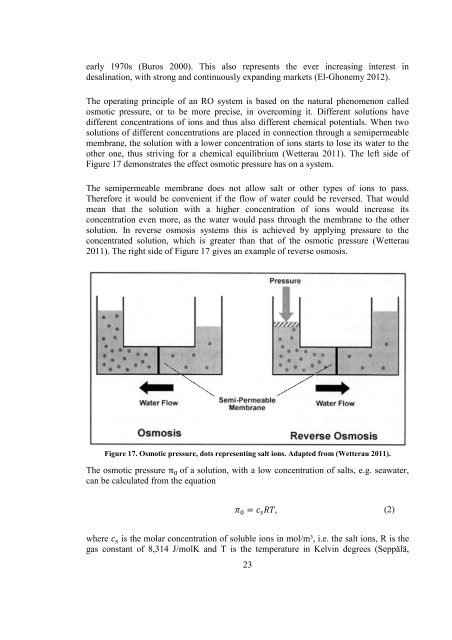
The operating principle of an RO system is based on the natural phenomenon called<br />
osmotic pressure, or to be more precise, in overcoming it. Different solutions have<br />
different concentrations of ions and thus also different chemical potentials. When two<br />
solutions of different concentrations are placed in connection through a semipermeable<br />
membrane, the solution with a lower concentration of ions starts to lose its water to the<br />
other one, thus striving for a chemical equilibrium (Wetterau 2011). The left side of<br />
Figure 17 demonstrates the effect osmotic pressure has on a system.<br />
The semipermeable membrane does not allow salt or other types of ions to pass.<br />
Therefore it would be convenient if the flow of water could be reversed. That would<br />
mean that the solution with a higher concentration of ions would increase its<br />
concentration even more, as the water would pass through the membrane to the other<br />
solution. In reverse osmosis systems this is achieved by applying pressure to the<br />
concentrated solution, which is greater than that of the osmotic pressure (Wetterau<br />
2011). The right side of Figure 17 gives an example of reverse osmosis.<br />
Figure 17. Osmotic pressure, dots representing salt ions. Adapted from (Wetterau 2011).<br />
The osmotic pressure of a solution, with a low concentration of salts, e.g. seawater,<br />
can be calculated from the equation<br />
where is the molar concentration of soluble ions in mol/m³, i.e. the salt ions, R is the<br />
gas constant of 8,314 J/molK and T is the temperature in Kelvin degrees (Seppälä,<br />
23<br />
(2)