1 THE OSMOMAT 070 VAPOR PRESSURE OSMOMETER ...
1 THE OSMOMAT 070 VAPOR PRESSURE OSMOMETER ...
1 THE OSMOMAT 070 VAPOR PRESSURE OSMOMETER ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
48 User Guide <strong>OSMOMAT</strong> <strong>070</strong> Version 1.1<br />
6 APPENDIX<br />
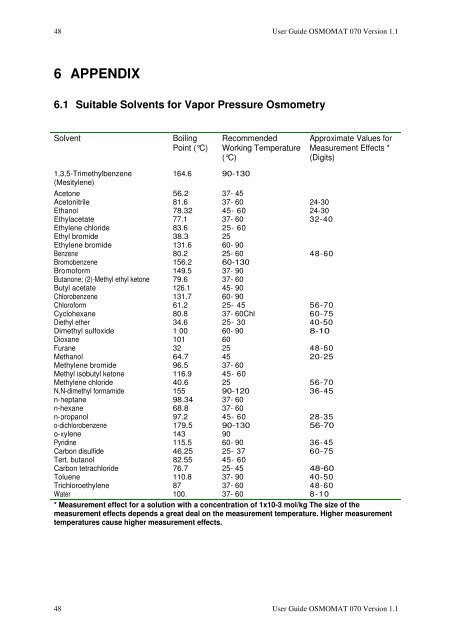
6.1 Suitable Solvents for Vapor Pressure Osmometry<br />
Solvent Boiling<br />
Point (°C)<br />
1,3,5-Trimethylbenzene<br />
(Mesitylene)<br />
164.6 90-130<br />
Recommended<br />
Working Temperature<br />
(°C)<br />
Approximate Values for<br />
Measurement Effects *<br />
(Digits)<br />
Acetone 56.2 37- 45<br />
Acetonitrile 81.6 37- 60 24-30<br />
Ethanol 78.32 45- 60 24-30<br />
Ethylacetate 77.1 37- 60 32-40<br />
Ethylene chloride 83.6 25- 60<br />
Ethyl bromide 38.3 25<br />
Ethylene bromide 131.6 60- 90<br />
Benzene 80.2 25- 60 48-60<br />
Bromobenzene 156.2 60-130<br />
Bromoform 149.5 37- 90<br />
Butanone; (2)-Methyl ethyl ketone 79.6 37- 60<br />
Butyl acetate 126.1 45- 90<br />
Chlorobenzene 131.7 60- 90<br />
Chloroform 61.2 25- 45 56-70<br />
Cyclohexane 80.8 37- 60Chl 60-75<br />
Diethyl ether 34.6 25- 30 40-50<br />
Dimethyl sulfoxide 1 00 60- 90 8-10<br />
Dioxane 101 60<br />
Furane 32 25 48-60<br />
Methanol 64.7 45 20-25<br />
Methylene bromide 96.5 37- 60<br />
Methyl isobutyl ketone 116.9 45- 60<br />
Methylene chloride 40.6 25 56-70<br />
N,N-dimethyl formamide 155 90-120 36-45<br />
n-heptane 98.34 37- 60<br />
n-hexane 68.8 37- 60<br />
n-propanol 97.2 45- 60 28-35<br />
o-dichlorobenzene 179.5 90-130 56-70<br />
o-xylene 143 90<br />
Pyridine 115.5 60- 90 36-45<br />
Carbon disulfide 46.25 25- 37 60-75<br />
Tert. butanol 82.55 45- 60<br />
Carbon tetrachloride 76.7 25- 45 48-60<br />
Toluene 110.8 37- 90 40-50<br />
Trichloroethylene 87 37- 60 48-60<br />
Water 100. 37- 60 8-10<br />
* Measurement effect for a solution with a concentration of 1x10-3 mol/kg The size of the<br />
measurement effects depends a great deal on the measurement temperature. Higher measurement<br />
temperatures cause higher measurement effects.<br />
48 User Guide <strong>OSMOMAT</strong> <strong>070</strong> Version 1.1