W0 2011/000721 A1 I||||||||||||||||||||||||||||||||||||||||||||||||||||||||| - Questel
W0 2011/000721 A1 I||||||||||||||||||||||||||||||||||||||||||||||||||||||||| - Questel
W0 2011/000721 A1 I||||||||||||||||||||||||||||||||||||||||||||||||||||||||| - Questel
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
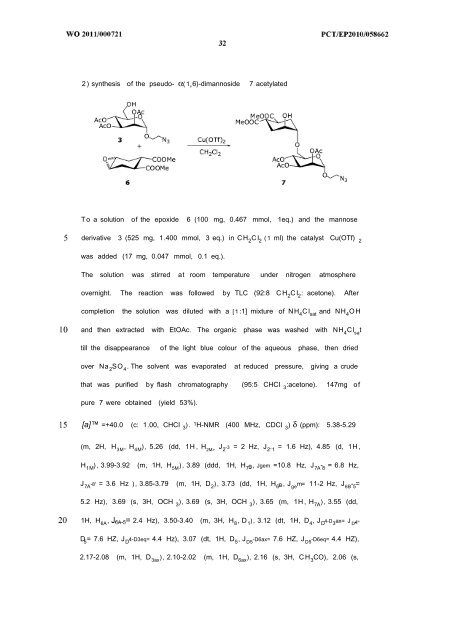
2 ) synthesis of the pseudo- α( 1 6)-dimannoside 7 acetylated<br />
T o a solution of the epoxide 6 (100 mg, 0.467 mmol, 1eq.) and the mannose<br />
derivative 3 (525 mg, 1.400 mmol, 3 eq.) in CH 2 C I 2 ( 1 ml) the catalyst Cu(OTf) 2<br />
was added (17 mg, 0.047 mmol, 0.1 eq.).<br />
The solution was stirred at room temperature under nitrogen atmosphere<br />
overnight. The reaction was followed by TLC (92:8 C H 2 CI 2 : acetone). After<br />
completion the solution was diluted with a [ 1 :1] mixture of NH 4 C l sat and NH 4 O H<br />
and then extracted with EtOAc. The organic phase was washed with NH 4 Cl sa t<br />
till the disappearance of the light blue colour of the aqueous phase, then dried<br />
over Na 2 SO 4 . The solvent was evaporated at reduced pressure, giving a crude<br />
that was purified by flash chromatography (95:5 CHCI 3 :acetone). 147mg of<br />
pure 7 were obtained (yield 53%).<br />
[a] =+40.0 (c: 1.00, CHCI 3 ). 1H-NMR (400 MHz, CDCI 3 ) δ (ppm): 5.38-5.29<br />
(m, 2H, H 3M , H 4M ), 5.26 (dd, 1H , H 2M> J 2 -3 = 2 Hz, J 2 1 = 1.6 Hz), 4.85 (d, 1H ,<br />
H 1M ), 3.99-3.92 (m, 1H, H 5M ), 3.89 (ddd, 1H, H 7 , Jgem =10.8 Hz, J 7A - 8 = 6.8 Hz,<br />
J 7A -8' = 3.6 Hz ), 3.85-3.79 (m, 1H, D 2 ), 3.73 (dd, 1H, H 6 , J ge m= 11-2 Hz, J 6B - 5 =<br />
5.2 Hz), 3.69 (s, 3H, OCH 3 ), 3.69 (s, 3H, OCH 3 ), 3.65 (m, 1H , H 7A ), 3.55 (dd,<br />
1H, H 6A , 2.4 Hz), 3.50-3.40 (m, 3H, H 8 , D 1 ), 3.12 (dt, 1H, D 4 , J D 4-D 3 ax= J D -<br />
D 5 = 7.6 HZ, J D 4-D3eq= 4.4 Hz), 3.07 (dt, 1H, D 5 , J D5 -D6ax= 7.6 HZ, J D5 -D6eq= 4.4 HZ),<br />
2.17-2.08 (m, 1H, D 3ax ), 2.10-2.02 (m, 1H, D 6ax ), 2.16 (s, 3H, C H 3 CO), 2.06 (s,