W0 2011/000721 A1 I||||||||||||||||||||||||||||||||||||||||||||||||||||||||| - Questel
W0 2011/000721 A1 I||||||||||||||||||||||||||||||||||||||||||||||||||||||||| - Questel
W0 2011/000721 A1 I||||||||||||||||||||||||||||||||||||||||||||||||||||||||| - Questel
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
(C2 D ), 70.36 (C6 M ), 70.01 (C6 M ), 68.68, 68.59 (C5 M C5 M ), 63.01 (C7), 52.36<br />
(OCH 3 ), 42.13 (C 8 ), 40.39 (C4 D ), 40.15 (C5 D ), 28.91 (C6 D ), 28.34 (C3 D ). MS<br />
(ESI) : Calculated for [C 24 H 42 NOi 6 + = 600.25, experimental = 600.3.<br />
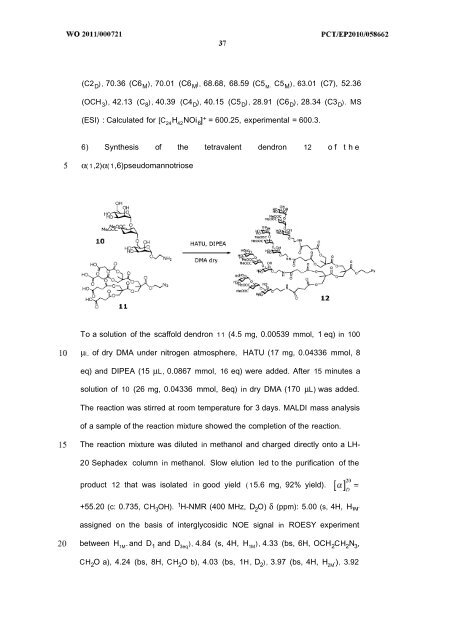
6) Synthesis of the tetravalent dendron 12 o f t h e<br />
α( 1 ,2)α( 1,6)pseudomannotriose<br />
To a solution of the scaffold dendron 1 1 (4.5 mg, 0.00539 mmol, 1 eq) in 100<br />
μl_ of dry DMA under nitrogen atmosphere, HATU (17 mg, 0.04336 mmol, 8<br />
eq) and DIPEA (15 μL, 0.0867 mmol, 16 eq) were added. After 15 minutes a<br />
solution of 10 (26 mg, 0.04336 mmol, 8eq) in dry DMA (170 μL) was added.<br />
The reaction was stirred at room temperature for 3 days. MALDI mass analysis<br />
of a sample of the reaction mixture showed the completion of the reaction.<br />
The reaction mixture was diluted in methanol and charged directly onto a LH-<br />
20 Sephadex column in methanol. Slow elution led to the purification of the<br />
product 12 that was isolated in good yield ( 15.6 mg, 92% yield).<br />
+55.20 (c: 0.735, CH 3 OH). 1 H-NMR (400 MHz, D 2 O) δ (ppm): 5.00 (s, 4H, H<br />
assigned on the basis of interglycosidic NOE signal in ROESY experiment<br />
between H 1M . and D 1 and D 3eq ), 4.84 (s, 4H, H 1M ), 4.33 (bs, 6H, OCH 2 CH 2 N 3 ,<br />
CH 2 O a), 4.24 (bs, 8H, CH 2 O b), 4.03 (bs, 1H , D 2 ), 3.97 (bs, 4H, H 2M , 3.92