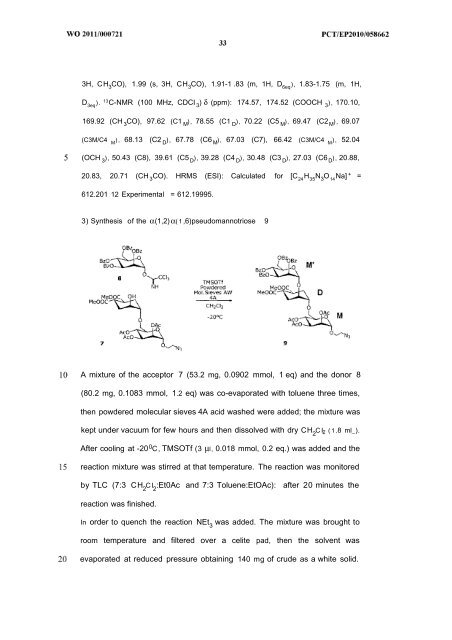
3H, CH 3 CO), 1.99 (s, 3H, CH 3 CO), 1.91-1 .83 (m, 1H, D 6eq ), 1.83-1.75 (m, 1H, D 3eq ). 13 C-NMR (100 MHz, CDCI 3 ) δ (ppm): 174.57, 174.52 (COOCH 3 ), 170.10, 169.92 (CH 3 CO), 97.62 (C1 M ), 78.55 (C1 D ), 70.22 (C5 M ), 69.47 (C2 M ), 69.07 (C3M/C4 M ) , 68.13 (C2 D ), 67.78 (C6 M ), 67.03 (C7), 66.42 (C3M/C4 M ), 52.04 (OCH 3 ), 50.43 (C8), 39.61 (C5 D ), 39.28 (C4 D ), 30.48 (C3 D ), 27.03 (C6 D ), 20.88, 20.83, 20.71 (CH 3 CO). HRMS (ESI): Calculated for [C 24 H 35 N 3 O 14 Na] + = 612.201 12 Experimental = 612.19995. 3) Synthesis of the α(1,2) α( 1 ,6)pseudomannotriose 9 A mixture of the acceptor 7 (53.2 mg, 0.0902 mmol, 1 eq) and the donor 8 (80.2 mg, 0.1083 mmol, 1.2 eq) was co-evaporated with toluene three times, then powdered molecular sieves 4A acid washed were added; the mixture was kept under vacuum for few hours and then dissolved with dry CH 2 C I ( 1 .8 ml_). After cooling at -20 0 C, TMSOTf (3 μl, 0.018 mmol, 0.2 eq.) was added and the reaction mixture was stirred at that temperature. The reaction was monitored by TLC (7:3 CH 2 CI 2 :Et0Ac and 7:3 Toluene:EtOAc): after 20 minutes the reaction was finished. In order to quench the reaction NEt 3 was added. The mixture was brought to room temperature and filtered over a celite pad, then the solvent was evaporated at reduced pressure obtaining 140 mg of crude as a white solid.
The purification was performed by flash chromatography (8:2 Toluene: EtOAc) leading to 9 (86 mg, 82%) as a white solid. [α ] ° =-2.16 (c: 1.016, CHCI 3 ). 1 H-NMR (400 MHz, CDCI 3 ) δ (ppm): 8.08 (dd, 4H, H-Ar OBz J = 6 Hz), 7.99 (d, 2H, H-Ar OBz J = 8 Hz), 7.84 (d, 2H, H-Ar OBz J = 8 Hz), 7.65-7.48 (m, 3H, H-Ar OBz), 7.47-7.34 (m, 7H, H-Ar OBz), 7.32-7.22 (m, 2H, H-Ar OBz), 6.14 (t, 1H, H 4 M-, J 3 = J 4-2 = 10.2 Hz), 5.88 (dd, 1H, H 3 M', J 3 -2 = 3.0 Hz), 5.70 (bs, 1H, H 2M ), 5.38 (dd, 1H, H 3M , J 3 - 2 = 4.0 Hz, J 3 4 = 10.0 Hz ), 5.34-5.23 (m, 2H, H 2M , H 4M ), 5.30 (s, 1H, H 1M -), 4.86 (s, 1H, H 1M ), 4.70 (dd, 1H H 6 BM J 6 - = 2.4 Hz, J gem = 12.0 Hz), 4.49 (dd, 1H, H 6AM , J 6 A- 5 = 4.4 Hz), 4.45-4.39 (m, 1H, H 5 M-). 4.10 (m, 1H , D 2 ), 3.99-3.92 (m, 1H , H 5M ), 3.92-3.85 (m, 1H, H 7B ), 3.76 (bs, 1H, D 1 ), 3.72-3.62 (m, 2H, H M , H 6BM ), 3.73 (s, 3H, OCH 3 ), 3.70 (s, 3H, OCH 3 ), 3.56 (dd, 1H, H 6A M . J g e m = 10.8 Hz, J 6A 5 = 2 Hz), 3.51-3.37 (m, 2H, H 8 ) , 3.09-2.98 (m, 2H, D 4 , D 5 ), 2.27-1.87 (m, 4H, D 3 , D 6 ), 2.16 (s, 3H, OCOCH 3 ), 2.06 (s, 3H, OCOCH 3 ), 2.00 (s, 3H, OCOCH 3 ). 13 C- NMR (100 MHz, CDCI 3 ) δ (ppm): 174.85, 174.76 (COOCH 3 ), 170.02, 169.84, 169.75 (COCH 3 ), 166.06, 165.49, 165.43, 165.37 (COPh), 133.56, 133.50, 133.23, 133.15 (CH Ar), 129.84, 129.72, 128.62, 128.47, 128.32 (CH Ar), 129.22, 128.99, 128.88 (C q Ar), 97.63 (C 1M ), 96.54 (C 1M ), 75.14 (C 02 ), 73.38 (CDI ), 70.80 (C 2 M-), 70.15 (C 5M ) , 69.81, (C 3 M-), 69.60 (C 5M ), 69.44 (C 4M ), 69.13 (C 3M ), 68.18 (C 6M ) , 66.99 (C 7 ) , 66.87 (C 4M ), 66.41 (C 3M ), 62.81 (C 6M ), 5 1.97, 51.91 (COOCH 3 ), 50.37 (C 8 ), 38.95, 38.74 (C 4 , C 05 ), 27.44, 27.28 (C 03 , C 06 ), 20.83, 20.77, 20.67 (OCOCH 3 ). MS (ESI) : [C 58 H 6 1 N 3 O 23 Na] + = 1190.6 4) Synthesis of the α(1,2)α(1,6)pseudomannotriose 2b
- Page 1 and 2: (12) INTERNATIONAL APPLICATION PUBL
- Page 3 and 4: mannose disaccharide (Manα1-2Man),
- Page 5 and 6: It is a forth object of the inventi
- Page 7 and 8: M' is a saccharidic or pseudo-sacch
- Page 9 and 10: wherein Q and n are above disclosed
- Page 12 and 13: wherein R is as above disclosed,
- Page 14 and 15: may be made of gold, silver, Fe 2 O
- Page 16 and 17: Within the compound of formula (I)
- Page 18 and 19: wherein R and R 2 are as above repo
- Page 20 and 21: through the Y linker and n is 1 to
- Page 22 and 23: and (12a) 12b. As per a third aspec
- Page 24 and 25: H wherein Q is selected from — N
- Page 26 and 27: According to a second object of the
- Page 28 and 29: wherein aj is 32 and J is a third g
- Page 30 and 31: eactions requiring anhydrous condit
- Page 32 and 33: To a solution of the silylmannopyra
- Page 36 and 37: To a solution of the compound 9 (12
- Page 38 and 39: (C2 D ), 70.36 (C6 M ), 70.01 (C6 M
- Page 40 and 41: EXAMPLE 2 Synthesis of compound 12a
- Page 42 and 43: 13 C-NMR (100 MHz, D2 O) δ (ppm):
- Page 44 and 45: (the R5 tropic laboratory-adapted s
- Page 46 and 47: derived from 1 (see above); G3Man32
- Page 48 and 49: CLAIMS: 1 A compound of general for
- Page 50: OCH 3 ), hydroxymethyl (-CH 2 OH),
- Page 53 and 54: alkyl chain, C1-C8 linear or branch
- Page 55 and 56: 7 . A compound having the general f
- Page 57 and 58: H wherein Q is selected from — N
- Page 59: or methyl substituted 1,3-thiazole;
- Page 62 and 63: wherein R 2 may be hydroxyl (-0H),
- Page 64 and 65: 12. A compound according to claim 1
- Page 66: or methyl substituted 1,3-thiazole;
- Page 82 and 83: INTERNATIONAL SEARCH REPORT C(Conti