W0 2011/000721 A1 I||||||||||||||||||||||||||||||||||||||||||||||||||||||||| - Questel
W0 2011/000721 A1 I||||||||||||||||||||||||||||||||||||||||||||||||||||||||| - Questel
W0 2011/000721 A1 I||||||||||||||||||||||||||||||||||||||||||||||||||||||||| - Questel
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
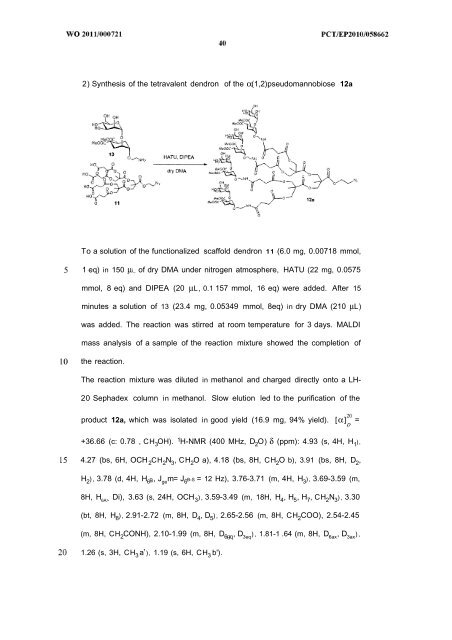
2) Synthesis of the tetravalent dendron of the α(1,2)pseudomannobiose 12a<br />
To a solution of the functionalized scaffold dendron 1 1 (6.0 mg, 0.00718 mmol,<br />
1 eq) in 150 μi_ of dry DMA under nitrogen atmosphere, HATU (22 mg, 0.0575<br />
mmol, 8 eq) and DIPEA (20 μL , 0.1 157 mmol, 16 eq) were added. After 15<br />
minutes a solution of 13 (23.4 mg, 0.05349 mmol, 8eq) in dry DMA (210 μL)<br />
was added. The reaction was stirred at room temperature for 3 days. MALDI<br />
mass analysis of a sample of the reaction mixture showed the completion of<br />
the reaction.<br />
The reaction mixture was diluted in methanol and charged directly onto a LH-<br />
20 Sephadex column in methanol. Slow elution led to the purification of the<br />
product 12a, which was isolated in good yield (16.9 mg, 94% yield). [α] =<br />
+36.66 (c: 0.78 , CH 3 OH). 1 H-NMR (400 MHz, D 2 O) δ (ppm): 4.93 (s, 4H, H 1 ),<br />
4.27 (bs, 6H, OCH 2 CH 2 N 3 , CH 2 O a), 4.18 (bs, 8H, CH 2 O b), 3.91 (bs, 8H, D 2 ,<br />
H 2 ), 3.78 (d, 4H, H 6 , J ge m= J 6 B-S = 12 Hz), 3.76-3.71 (m, 4H, H 3 ) , 3.69-3.59 (m,<br />
8H, H 6A> Di), 3.63 (s, 24H, OCH 3 ), 3.59-3.49 (m, 18H, H 4 , H 5 , H 7 , CH 2 N 3 ), 3.30<br />
(bt, 8H, H 8 ), 2.91-2.72 (m, 8H, D 4 , D 5 ), 2.65-2.56 (m, 8H, CH 2 COO), 2.54-2.45<br />
(m, 8H, CH 2 CONH), 2.10-1.99 (m, 8H, D 6θq , D 3eq ), 1.81-1 .64 (m, 8H, D 6ax , D 3ax ),<br />
1.26 (s, 3H, CH 3 a ), 1.19 (s, 6H, CH 3 b').