Vaporization of JP-8 Jet Fuel in a Simulated Aircraft Fuel Tank ...
Vaporization of JP-8 Jet Fuel in a Simulated Aircraft Fuel Tank ...
Vaporization of JP-8 Jet Fuel in a Simulated Aircraft Fuel Tank ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Temperature, Deg. F.<br />
40<br />
35<br />
30<br />
25<br />
20<br />
15<br />
10<br />
5<br />
0<br />
Thermocouple<br />
Error<br />
FID Error<br />
<strong>Fuel</strong> Temp<br />
Measured THC<br />
Calculated THC<br />
0 1000 2000 3000 4000 5000 6000<br />
Time, seconds<br />
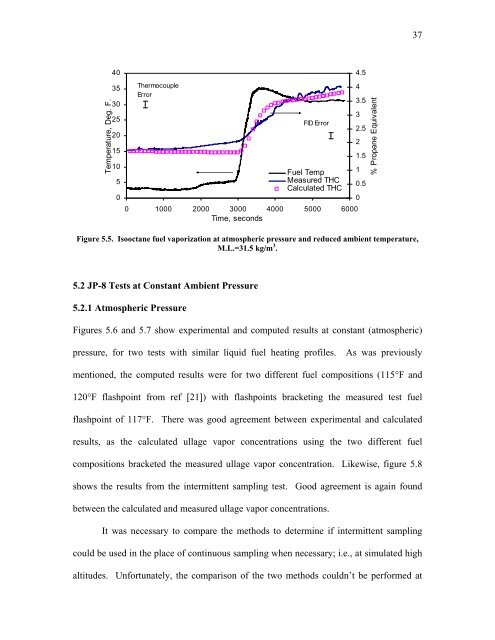
Figure 5.5. Isooctane fuel vaporization at atmospheric pressure and reduced ambient temperature,<br />
M.L.=31.5 kg/m 3 .<br />
5.2 <strong>JP</strong>-8 Tests at Constant Ambient Pressure<br />
5.2.1 Atmospheric Pressure<br />
Figures 5.6 and 5.7 show experimental and computed results at constant (atmospheric)<br />
pressure, for two tests with similar liquid fuel heat<strong>in</strong>g pr<strong>of</strong>iles. As was previously<br />
mentioned, the computed results were for two different fuel compositions (115°F and<br />
120°F flashpo<strong>in</strong>t from ref [21]) with flashpo<strong>in</strong>ts bracket<strong>in</strong>g the measured test fuel<br />
flashpo<strong>in</strong>t <strong>of</strong> 117°F. There was good agreement between experimental and calculated<br />
results, as the calculated ullage vapor concentrations us<strong>in</strong>g the two different fuel<br />
compositions bracketed the measured ullage vapor concentration. Likewise, figure 5.8<br />
shows the results from the <strong>in</strong>termittent sampl<strong>in</strong>g test. Good agreement is aga<strong>in</strong> found<br />
between the calculated and measured ullage vapor concentrations.<br />
It was necessary to compare the methods to determ<strong>in</strong>e if <strong>in</strong>termittent sampl<strong>in</strong>g<br />
could be used <strong>in</strong> the place <strong>of</strong> cont<strong>in</strong>uous sampl<strong>in</strong>g when necessary; i.e., at simulated high<br />
altitudes. Unfortunately, the comparison <strong>of</strong> the two methods couldn’t be performed at<br />
4.5<br />
4<br />
3.5<br />
3<br />
2.5<br />
2<br />
1.5<br />
1<br />
0.5<br />
0<br />
% Propane Equivalent<br />
37