Chemical and toxicological properties of coal fly ash - University of ...
Chemical and toxicological properties of coal fly ash - University of ...
Chemical and toxicological properties of coal fly ash - University of ...
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
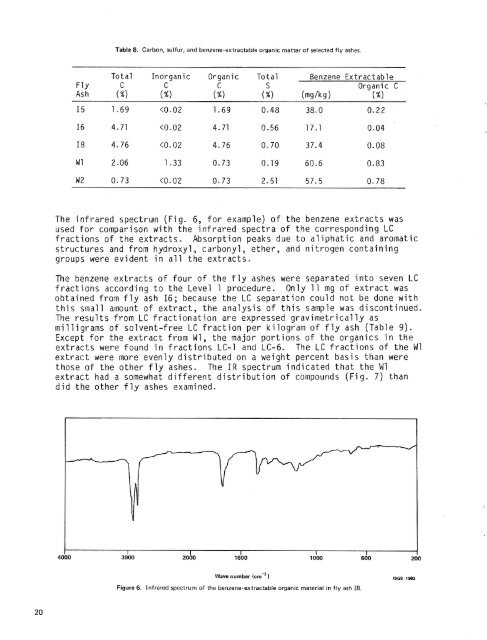
Table 8. Carbon, sulfur, <strong>and</strong> benzene-extractable organic matter <strong>of</strong> selected <strong>fly</strong> <strong>ash</strong>es.Total Inorganic Organic Total Benzene ExtractableFl Y C C C S Organic CAsh (%I (%) (%) (%I (mg/kd (%IThe infrared spectrum (Fig. 6, for example) <strong>of</strong> the benzene extracts wasused for comparison with the infrared spectra <strong>of</strong> the corresponding LCfractions <strong>of</strong> the extracts. Absorption peaks due to aliphatic <strong>and</strong> aromaticstructures <strong>and</strong> from hydroxyl, carbonyl, ether, <strong>and</strong> nitrogen containinggroups were evident in all the extracts.The benzene extracts <strong>of</strong> four <strong>of</strong> the <strong>fly</strong> <strong>ash</strong>es were separated into seven LCfractions according to the Level 1 procedure. Only 11 mg <strong>of</strong> extract wasobtained from <strong>fly</strong> <strong>ash</strong> 16; because the LC separation could not be done withthis small amount <strong>of</strong> extract, the analysis <strong>of</strong> this sample was discontinued.The results from LC fractionation are expressed gravimetrically asmilligrams <strong>of</strong> solvent-free LC fraction per kilogram <strong>of</strong> <strong>fly</strong> <strong>ash</strong> (Table 9).Except for the extract from W1, the major portions <strong>of</strong> the organics in theextracts were found in fractions LC-1 <strong>and</strong> LC-6. The LC fractions <strong>of</strong> the W1extract were more evenly distributed on a weight percent basis than werethose <strong>of</strong> the other <strong>fly</strong> <strong>ash</strong>es. The IR spectrum indicated that the W1extract had a somewhat different distribution <strong>of</strong> compounds (Fig. 7) th<strong>and</strong>id the other <strong>fly</strong> <strong>ash</strong>es examined.aoQo 2000 lajb0 1000 sdoWave number (cm-' 1Figure 6. Infrared spectrum <strong>of</strong> the benzene-extractable organic material in <strong>fly</strong> <strong>ash</strong> 18.