Chemical and toxicological properties of coal fly ash - University of ...
Chemical and toxicological properties of coal fly ash - University of ...
Chemical and toxicological properties of coal fly ash - University of ...
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
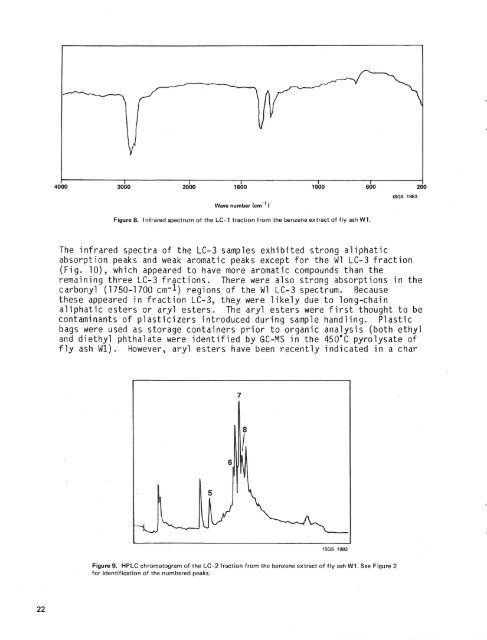
Wave number (cm-'Figure 8. Infrared spectrum <strong>of</strong> the LC- 1 fraction from the benzene extract <strong>of</strong> <strong>fly</strong> <strong>ash</strong> W1.The infrared spectra <strong>of</strong> the LC-3 samples exhibited strong aliphaticabsorption peaks <strong>and</strong> weak aromatic peaks except for the W1 LC-3 fraction(Fig. lo), which appeared to have more aromatic compounds than theremaining three LC-3 fractions. There were also strong absorptions in thecarbonyl (1750-1 700 cm-1) regions <strong>of</strong> the W1 LC-3 spectrum. Becausethese appeared in fraction LC-3, they were likely due to long-chainaliphatic esters or aryl esters. The aryl esters were first thought to becontaminants <strong>of</strong> plasticizers introduced during sample h<strong>and</strong>ling. Plasticbags were used as storage containers prior to organic analysis (both ethyl<strong>and</strong> diethyl phthalate were identified by GC-YS in the 450°C pyrolysate <strong>of</strong><strong>fly</strong> <strong>ash</strong> Wl). However, aryl esters have been recently indicated in a charFigure 9. HPLC chromatogram <strong>of</strong> the LC-2 fraction from the benzene extract <strong>of</strong> <strong>fly</strong> <strong>ash</strong> W1. See Figure 2for identification <strong>of</strong> the numbered peaks.