SOP â Malaria Microscopy - NVBDCP
SOP â Malaria Microscopy - NVBDCP
SOP â Malaria Microscopy - NVBDCP
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
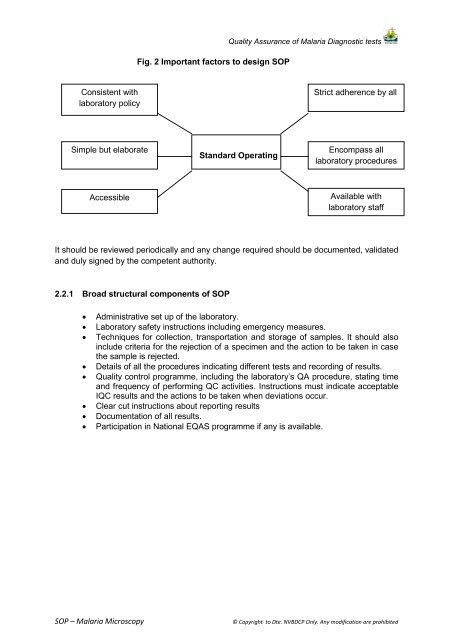
Fig. 2 Important factors to design <strong>SOP</strong>Quality Assurance of <strong>Malaria</strong> Diagnostic testsConsistent withlaboratory policyStrict adherence by allSimple but elaborateStandard OperatingEncompass alllaboratory proceduresAccessibleAvailable withlaboratory staffIt should be reviewed periodically and any change required should be documented, validatedand duly signed by the competent authority.2.2.1 Broad structural components of <strong>SOP</strong>• Administrative set up of the laboratory.• Laboratory safety instructions including emergency measures.• Techniques for collection, transportation and storage of samples. It should alsoinclude criteria for the rejection of a specimen and the action to be taken in casethe sample is rejected.• Details of all the procedures indicating different tests and recording of results.• Quality control programme, including the laboratory’s QA procedure, stating timeand frequency of performing QC activities. Instructions must indicate acceptableIQC results and the actions to be taken when deviations occur.• Clear cut instructions about reporting results• Documentation of all results.• Participation in National EQAS programme if any is available.<strong>SOP</strong> – <strong>Malaria</strong> <strong>Microscopy</strong>© Copyright to Dte. <strong>NVBDCP</strong> Only. Any modification are prohibited