SOP â Malaria Microscopy - NVBDCP
SOP â Malaria Microscopy - NVBDCP
SOP â Malaria Microscopy - NVBDCP
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
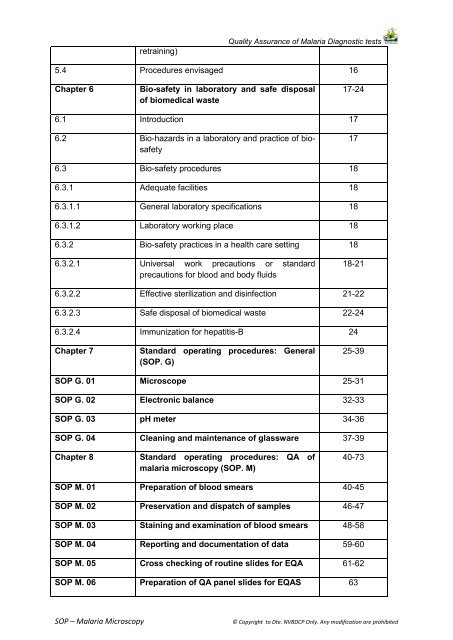
etraining)Quality Assurance of <strong>Malaria</strong> Diagnostic tests5.4 Procedures envisaged 16Chapter 6Bio-safety in laboratory and safe disposalof biomedical waste17-246.1 Introduction 176.2 Bio-hazards in a laboratory and practice of biosafety176.3 Bio-safety procedures 186.3.1 Adequate facilities 186.3.1.1 General laboratory specifications 186.3.1.2 Laboratory working place 186.3.2 Bio-safety practices in a health care setting 186.3.2.1 Universal work precautions or standardprecautions for blood and body fluids18-216.3.2.2 Effective sterilization and disinfection 21-226.3.2.3 Safe disposal of biomedical waste 22-246.3.2.4 Immunization for hepatitis-B 24Chapter 7Standard operating procedures: General(<strong>SOP</strong>. G)25-39<strong>SOP</strong> G. 01 Microscope 25-31<strong>SOP</strong> G. 02 Electronic balance 32-33<strong>SOP</strong> G. 03 pH meter 34-36<strong>SOP</strong> G. 04 Cleaning and maintenance of glassware 37-39Chapter 8Standard operating procedures: QA ofmalaria microscopy (<strong>SOP</strong>. M)40-73<strong>SOP</strong> M. 01 Preparation of blood smears 40-45<strong>SOP</strong> M. 02 Preservation and dispatch of samples 46-47<strong>SOP</strong> M. 03 Staining and examination of blood smears 48-58<strong>SOP</strong> M. 04 Reporting and documentation of data 59-60<strong>SOP</strong> M. 05 Cross checking of routine slides for EQA 61-62<strong>SOP</strong> M. 06 Preparation of QA panel slides for EQAS 63<strong>SOP</strong> – <strong>Malaria</strong> <strong>Microscopy</strong>© Copyright to Dte. <strong>NVBDCP</strong> Only. Any modification are prohibited