WHO Drug Information Vol. 25, No. 2, 2011
WHO Drug Information Vol. 25, No. 2, 2011
WHO Drug Information Vol. 25, No. 2, 2011
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
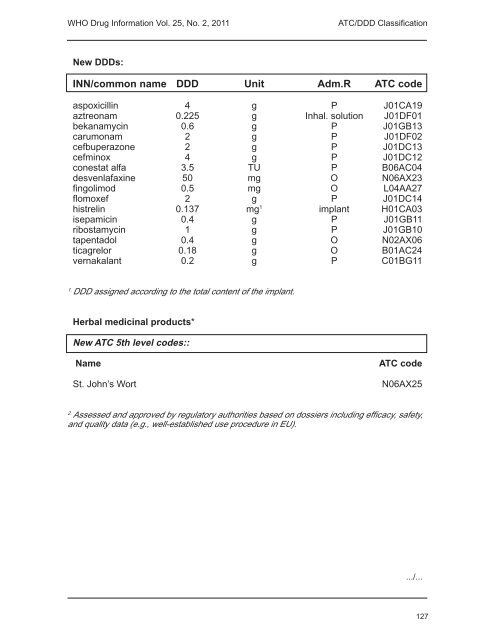
<strong>WHO</strong> <strong>Drug</strong> <strong>Information</strong> <strong>Vol</strong>. <strong>25</strong>, <strong>No</strong>. 2, <strong>2011</strong>ATC/DDD ClassificationNew DDDs:INN/common name DDD Unit Adm.R ATC codeaspoxicillin 4 g P J01CA19aztreonam 0.2<strong>25</strong> g Inhal. solution J01DF01bekanamycin 0.6 g P J01GB13carumonam 2 g P J01DF02cefbuperazone 2 g P J01DC13cefminox 4 g P J01DC12conestat alfa 3.5 TU P B06AC04desvenlafaxine 50 mg O N06AX23fingolimod 0.5 mg O L04AA27flomoxef 2 g P J01DC14histrelin 0.137 mg 1 implant H01CA03isepamicin 0.4 g P J01GB11ribostamycin 1 g P J01GB10tapentadol 0.4 g O N02AX06ticagrelor 0.18 g O B01AC24vernakalant 0.2 g P C01BG111.DDD assigned according to the total content of the implant.Herbal medicinal products*New ATC 5th level codes::NameSt. John’s WortATC codeN06AX<strong>25</strong>2.Assessed and approved by regulatory authorities based on dossiers including efficacy, safety,and quality data (e.g., well-established use procedure in EU)..../...127