WHO Drug Information Vol. 24, No. 4, 2010
WHO Drug Information Vol. 24, No. 4, 2010
WHO Drug Information Vol. 24, No. 4, 2010
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
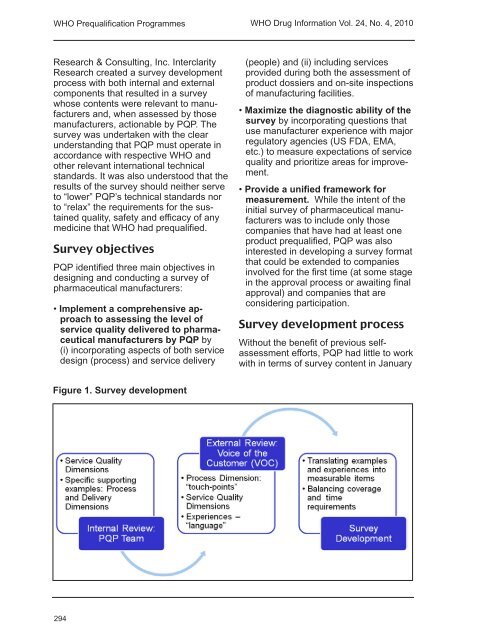
<strong>WHO</strong> Prequalification Programmes<strong>WHO</strong> <strong>Drug</strong> <strong>Information</strong> <strong>Vol</strong>. <strong>24</strong>, <strong>No</strong>. 4, <strong>2010</strong>Research & Consulting, Inc. InterclarityResearch created a survey developmentprocess with both internal and externalcomponents that resulted in a surveywhose contents were relevant to manufacturersand, when assessed by thosemanufacturers, actionable by PQP. Thesurvey was undertaken with the clearunderstanding that PQP must operate inaccordance with respective <strong>WHO</strong> andother relevant international technicalstandards. It was also understood that theresults of the survey should neither serveto “lower” PQP’s technical standards norto “relax” the requirements for the sustainedquality, safety and efficacy of anymedicine that <strong>WHO</strong> had prequalified.Survey objectivesPQP identified three main objectives indesigning and conducting a survey ofpharmaceutical manufacturers:• Implement a comprehensive approachto assessing the level ofservice quality delivered to pharmaceuticalmanufacturers by PQP by(i) incorporating aspects of both servicedesign (process) and service delivery(people) and (ii) including servicesprovided during both the assessment ofproduct dossiers and on-site inspectionsof manufacturing facilities.• Maximize the diagnostic ability of thesurvey by incorporating questions thatuse manufacturer experience with majorregulatory agencies (US FDA, EMA,etc.) to measure expectations of servicequality and prioritize areas for improvement.• Provide a unified framework formeasurement. While the intent of theinitial survey of pharmaceutical manufacturerswas to include only thosecompanies that have had at least oneproduct prequalified, PQP was alsointerested in developing a survey formatthat could be extended to companiesinvolved for the first time (at some stagein the approval process or awaiting finalapproval) and companies that areconsidering participation.Survey development processWithout the benefit of previous selfassessmentefforts, PQP had little to workwith in terms of survey content in JanuaryFigure 1. Survey development294