Association
Magnetic Oxide Heterostructures: EuO on Cubic Oxides ... - JuSER
Magnetic Oxide Heterostructures: EuO on Cubic Oxides ... - JuSER
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
5.1. Chemical stabilization of bulk-like EuO directly on silicon 93<br />
to the valency quantification of the oxygen-rich Eu compound (ii). Thus, we estimate the<br />
mixed-valent Eu 3 O 4 to be the origin of the Eu 2+ signal in oxygen-rich Eu 1 O 1+x , which may<br />
also explain the Eu 2+ accumulation near the surface as a result of reduction by metallic Al<br />
from the capping layer. The results of this section are published in Caspers et al. (2011). 1<br />
HAXPES: Analysis of the Eu 4s and Eu 4d core-levels<br />
In order to develop a consistent compositional analysis of the two complementary EuO/Si<br />
heterostructures (i) and (ii), we include the Eu 4s and 4d photoemission spectra into the<br />
quantitative analysis. In studying the 4s and 4d core-levels, one has to take into account<br />
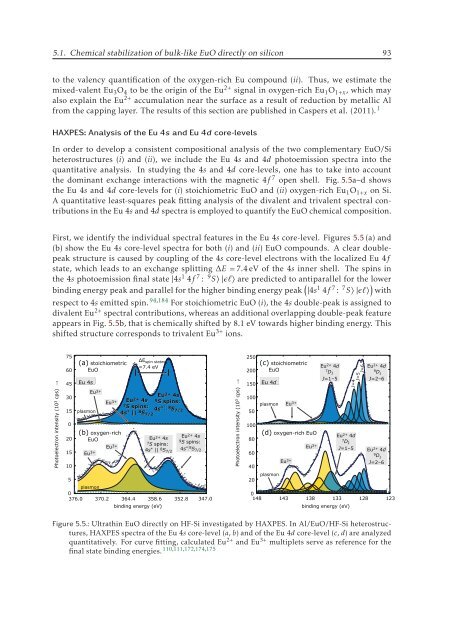
the dominant exchange interactions with the magnetic 4f 7 open shell. Fig. 5.5a–d shows<br />
the Eu 4s and 4d core-levels for (i) stoichiometric EuO and (ii) oxygen-rich Eu 1 O 1+x on Si.<br />
A quantitative least-squares peak fitting analysis of the divalent and trivalent spectral contributions<br />
in the Eu 4s and 4d spectra is employed to quantify the EuO chemical composition.<br />
First, we identify the individual spectral features in the Eu 4s core-level. Figures 5.5 (a) and<br />
(b) show the Eu 4s core-level spectra for both (i) and (ii) EuO compounds. A clear doublepeak<br />
structure is caused by coupling of the 4s core-level electrons with the localized Eu 4f<br />
state, which leads to an exchange splitting ΔE = 7.4 eV of the 4s inner shell. The spins in<br />
the 4s photoemission final state |4s 1 4f 7 : 9 S〉|ɛl〉 are predicted to antiparallel for the lower<br />
binding energy peak and parallel for the higher binding energy peak ( |4s 1 4f 7 : 7 S〉|ɛl〉 ) with<br />
respect to 4s emitted spin. 94,184 For stoichiometric EuO (i), the 4s double-peak is assigned to<br />
divalent Eu 2+ spectral contributions, whereas an additional overlapping double-peak feature<br />
appears in Fig. 5.5b, that is chemically shifted by 8.1 eV towards higher binding energy. This<br />
shifted structure corresponds to trivalent Eu 3+ ions.<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
Figure 5.5.: Ultrathin EuO directly on HF-Si investigated by HAXPES. In Al/EuO/HF-Si heterostructures,<br />
HAXPES spectra of the Eu 4s core-level (a, b) and of the Eu 4d core-level (c, d) are analyzed<br />
quantitatively. For curve fitting, calculated Eu 2+ and Eu 3+ multiplets serve as reference for the<br />
final state binding energies. 110,111,172,174,175