Association
Magnetic Oxide Heterostructures: EuO on Cubic Oxides ... - JuSER
Magnetic Oxide Heterostructures: EuO on Cubic Oxides ... - JuSER
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
4.3. Lateral compressive strain: EuO on MgO (100) 81<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
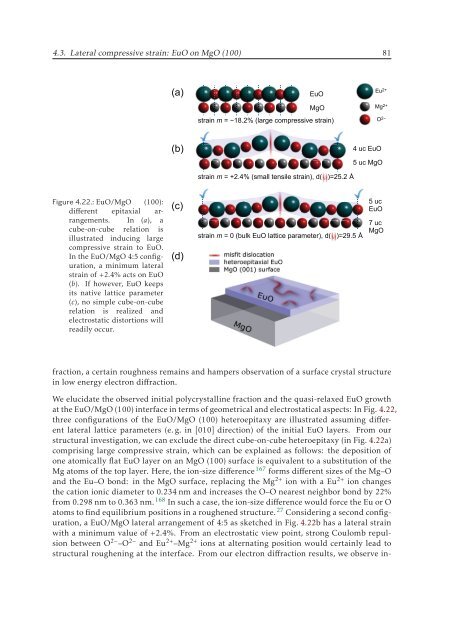
Figure 4.22.: EuO/MgO (100):<br />
different epitaxial arrangements.<br />
In (a), a<br />
cube-on-cube relation is<br />
illustrated inducing large<br />
compressive strain to EuO.<br />
In the EuO/MgO 4:5 configuration,<br />
a minimum lateral<br />
strain of +2.4% acts on EuO<br />
(b). If however, EuO keeps<br />
its native lattice parameter<br />
(c), no simple cube-on-cube<br />
relation is realized and<br />
electrostatic distortions will<br />
readily occur.<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
fraction, a certain roughness remains and hampers observation of a surface crystal structure<br />
in low energy electron diffraction.<br />
We elucidate the observed initial polycrystalline fraction and the quasi-relaxed EuO growth<br />
at the EuO/MgO (100) interface in terms of geometrical and electrostatical aspects: In Fig. 4.22,<br />
three configurations of the EuO/MgO (100) heteroepitaxy are illustrated assuming different<br />
lateral lattice parameters (e. g. in [010] direction) of the initial EuO layers. From our<br />
structural investigation, we can exclude the direct cube-on-cube heteroepitaxy (in Fig. 4.22a)<br />
comprising large compressive strain, which can be explained as follows: the deposition of<br />
one atomically flat EuO layer on an MgO (100) surface is equivalent to a substitution of the<br />
Mg atoms of the top layer. Here, the ion-size difference 167 forms different sizes of the Mg–O<br />
and the Eu–O bond: in the MgO surface, replacing the Mg 2+ ion with a Eu 2+ ion changes<br />
the cation ionic diameter to 0.234 nm and increases the O–O nearest neighbor bond by 22%<br />
from 0.298 nm to 0.363 nm. 168 In such a case, the ion-size difference would force the Eu or O<br />
atoms to find equilibrium positions in a roughened structure. 27 Considering a second configuration,<br />
a EuO/MgO lateral arrangement of 4:5 as sketched in Fig. 4.22b has a lateral strain<br />
with a minimum value of +2.4%. From an electrostatic view point, strong Coulomb repulsion<br />
between O 2− –O 2− and Eu 2+ –Mg 2+ ions at alternating position would certainly lead to<br />
structural roughening at the interface. From our electron diffraction results, we observe in-