Association
Magnetic Oxide Heterostructures: EuO on Cubic Oxides ... - JuSER
Magnetic Oxide Heterostructures: EuO on Cubic Oxides ... - JuSER
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
98 5. Results II: EuO integration directly on silicon<br />
What is the free parameter which drives the system into one of these regimes? The phase<br />
rule for constant pressures (5.1) evaluates F = 1, a degree of freedom in only one dimension.<br />
This degree of freedom is the temperature of synthesis T S . Via this temperature, the Eu distillation<br />
condition during EuO synthesis is strongly affected by means of partial or complete<br />
re-evaporation of excess Eu, this corresponds to a shift along the O–Eu tie line in the Gibbs<br />
triangle. Therefore, the chemical phases can range from oxidized phases over stoichiometric<br />
EuO to metallic phases. This variation is plotted in Fig. 5.8b with different background colors<br />
revealing the chemical regimes (I), (II), and (III), all depending on the temperature degree of<br />
freedom.<br />
We proceed with the quantitative analysis of EuO/Si interfacial reactions products. The reactions<br />
are evaluated by balancing the Gibbs free energies of formation,<br />
ΔG ◦ =<br />
∑<br />
products<br />
nG ◦ f<br />
−<br />
∑<br />
reactants<br />
mGf ◦ . (after Hess’ law) (5.2)<br />
These balances are not limited to the comparison of bare formation energies G f (T ) directly<br />
from the constituents as presented in the Ellingham diagram. We rather compare oxygen-rich<br />
(I) with Eu-distillation (II–III) chemical regimes during the EuO synthesis in the initial stage<br />
and during sustained growth. Herein, the distillation condition is persistently expressed by<br />
the term (3Eu + O 2 ) and the oxygen-rich synthesis as (Eu + 3/2O 2 ). First, we address the native<br />
metallic silicide EuSi 2 , and then the silicon dioxide SiO 2 . Among ternary compounds, we<br />
limit the discussion to Eu(OH) 3 which is the most probable europium hydroxide. In the case<br />
of a two-dimensional structure, we remark that the energy gain ΔG ◦ is reduced compared to<br />
a volume reaction by the surface energy of the substrate: ΔG ◦ (1×1)<br />
= 113 kJ/mol for (1 × 1)-Si<br />
(001), or ΔG ◦ (2×1)<br />
= 124 kJ/mol for (2 × 1)-Si (001).190<br />
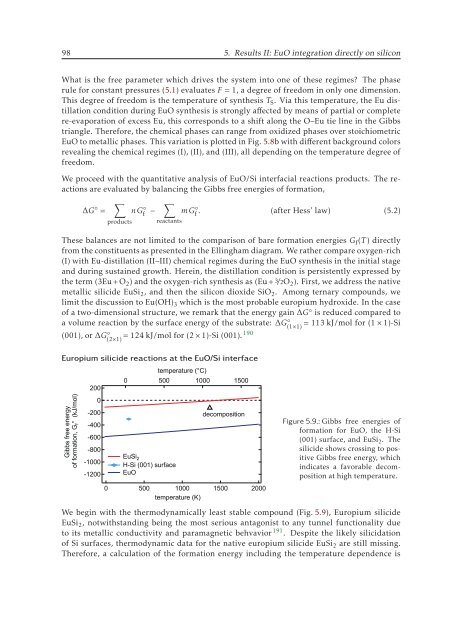
Europium silicide reactions at the EuO/Si interface<br />
temperature (°C)<br />
0 500 1000 1500<br />
200<br />
Gibbs free energy<br />
of formation, G f ° (kJ/mol)<br />
0<br />
-200<br />
-400<br />
-600<br />
-800<br />
-1000<br />
-1200<br />
0<br />
EuSi 2<br />
H-Si (001) surface<br />
EuO<br />
500 1000<br />
temperature (K)<br />
decomposition<br />
1500<br />
2000<br />
Figure 5.9.: Gibbs free energies of<br />
formation for EuO, the H-Si<br />
(001) surface, and EuSi 2 . The<br />
silicide shows crossing to positive<br />
Gibbs free energy, which<br />
indicates a favorable decomposition<br />
at high temperature.<br />
We begin with the thermodynamically least stable compound (Fig. 5.9), Europium silicide<br />
EuSi 2 , notwithstanding being the most serious antagonist to any tunnel functionality due<br />
to its metallic conductivity and paramagnetic behvavior 191 . Despite the likely silicidation<br />
of Si surfaces, thermodynamic data for the native europium silicide EuSi 2 are still missing.<br />
Therefore, a calculation of the formation energy including the temperature dependence is