Association
Magnetic Oxide Heterostructures: EuO on Cubic Oxides ... - JuSER
Magnetic Oxide Heterostructures: EuO on Cubic Oxides ... - JuSER
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
32 2. Theoretical background<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
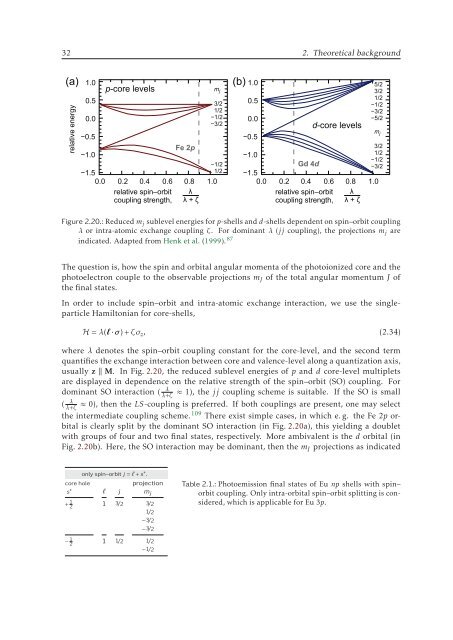
Figure 2.20.: Reduced m j sublevel energies for p-shells and d-shells dependent on spin–orbit coupling<br />
λ or intra-atomic exchange coupling ζ. For dominant λ (jj coupling), the projections m j are<br />
indicated. Adapted from Henk et al. (1999). 87<br />
The question is, how the spin and orbital angular momenta of the photoionized core and the<br />
photoelectron couple to the observable projections m J of the total angular momentum J of<br />
the final states.<br />
In order to include spin–orbit and intra-atomic exchange interaction, we use the singleparticle<br />
Hamiltonian for core-shells,<br />
H = λ(l·σ)+ζσ z , (2.34)<br />
where λ denotes the spin–orbit coupling constant for the core-level, and the second term<br />
quantifies the exchange interaction between core and valence-level along a quantization axis,<br />
usually z ‖ M. In Fig. 2.20, the reduced sublevel energies of p and d core-level multiplets<br />
are displayed in dependence on the relative strength of the spin–orbit (SO) coupling. For<br />
dominant SO interaction (<br />
λ+ζ λ ≈ 1), the jj coupling scheme is suitable. If the SO is small<br />
≈ 0), then the LS-coupling is preferred. If both couplings are present, one may select<br />
( λ<br />
λ+ζ<br />
the intermediate coupling scheme. 109 There exist simple cases, in which e. g. the Fe 2p orbital<br />
is clearly split by the dominant SO interaction (in Fig. 2.20a), this yielding a doublet<br />
with groups of four and two final states, respectively. More ambivalent is the d orbital (in<br />
Fig. 2.20b). Here, the SO interaction may be dominant, then the m J projections as indicated<br />
only spin–orbit j = + s ∗ .<br />
core hole<br />
projection<br />
s ∗ j m j<br />
+ 1 2 1 3/2 3/2<br />
1/2<br />
−3/2<br />
−3/2<br />
Table 2.1.: Photoemission final states of Eu np shells with spin–<br />
orbit coupling. Only intra-orbital spin–orbit splitting is considered,<br />
which is applicable for Eu 3p.<br />
− 1 2 1 1/2 1/2<br />
−1/2