Association
Magnetic Oxide Heterostructures: EuO on Cubic Oxides ... - JuSER
Magnetic Oxide Heterostructures: EuO on Cubic Oxides ... - JuSER
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
5.3. Interface engineering I: Hydrogen passivation of the EuO/Si interface 109<br />
110<br />
<br />
<br />
100<br />
90<br />
80<br />
70<br />
60<br />
50<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
102 101 100 99 98 97<br />
<br />
<br />
<br />
T S = 350 °C, Si<br />
T S = 450 °C, Si<br />
<br />
20<br />
T S = 350 °C<br />
pure Si<br />
0.65 nm<br />
silicide<br />
T S = 450 °C<br />
pure Si<br />
0.30 nm<br />
silicide<br />
50<br />
9.7% EuSi y<br />
<br />
T S = 350 °C, H-Si<br />
T S = 450 °C, H-Si<br />
<br />
10<br />
0<br />
50<br />
25<br />
T S = 350 °C<br />
H-Si<br />
0.56 nm<br />
silicide<br />
4.4% EuSi y<br />
T S = 450 °C<br />
H-Si<br />
0.14 nm<br />
silicide<br />
25<br />
0<br />
60<br />
30<br />
<br />
0<br />
0<br />
96.5<br />
102.5 100.5 98.5<br />
100.5 98.5 96.5<br />
102.5<br />
<br />
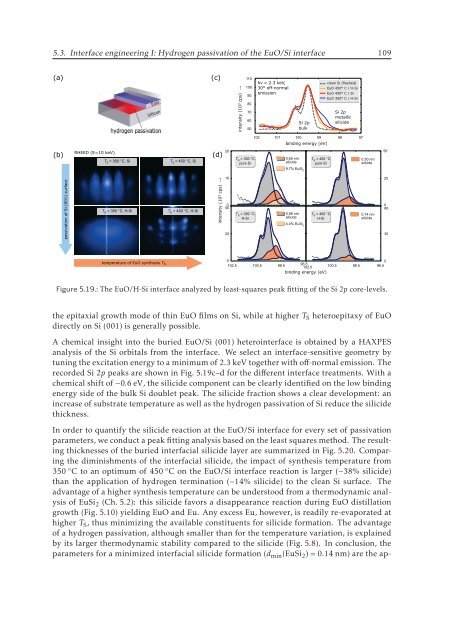
Figure 5.19.: The EuO/H-Si interface analyzed by least-squares peak fitting of the Si 2p core-levels.<br />
the epitaxial growth mode of thin EuO films on Si, while at higher T S heteroepitaxy of EuO<br />
directly on Si (001) is generally possible.<br />
A chemical insight into the buried EuO/Si (001) heterointerface is obtained by a HAXPES<br />
analysis of the Si orbitals from the interface. We select an interface-sensitive geometry by<br />
tuning the excitation energy to a minimum of 2.3 keV together with off-normal emission. The<br />
recorded Si 2p peaks are shown in Fig. 5.19c–d for the different interface treatments. With a<br />
chemical shift of −0.6 eV, the silicide component can be clearly identified on the low binding<br />
energy side of the bulk Si doublet peak. The silicide fraction shows a clear development: an<br />
increase of substrate temperature as well as the hydrogen passivation of Si reduce the silicide<br />
thickness.<br />
In order to quantify the silicide reaction at the EuO/Si interface for every set of passivation<br />
parameters, we conduct a peak fitting analysis based on the least squares method. The resulting<br />
thicknesses of the buried interfacial silicide layer are summarized in Fig. 5.20. Comparing<br />
the diminishments of the interfacial silicide, the impact of synthesis temperature from<br />
350 ◦ C to an optimum of 450 ◦ C on the EuO/Si interface reaction is larger (−38% silicide)<br />
than the application of hydrogen termination (−14% silicide) to the clean Si surface. The<br />
advantage of a higher synthesis temperature can be understood from a thermodynamic analysis<br />
of EuSi 2 (Ch. 5.2): this silicide favors a disappearance reaction during EuO distillation<br />
growth (Fig. 5.10) yielding EuO and Eu. Any excess Eu, however, is readily re-evaporated at<br />
higher T S , thus minimizing the available constituents for silicide formation. The advantage<br />
of a hydrogen passivation, although smaller than for the temperature variation, is explained<br />
by its larger thermodynamic stability compared to the silicide (Fig. 5.8). In conclusion, the<br />
parameters for a minimized interfacial silicide formation (d min (EuSi 2 ) = 0.14 nm) are the ap-