European Journal of Scientific Research - EuroJournals
European Journal of Scientific Research - EuroJournals
European Journal of Scientific Research - EuroJournals
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
335 S. J. Fatemi, A. Badiei and A. Hooshmand<br />
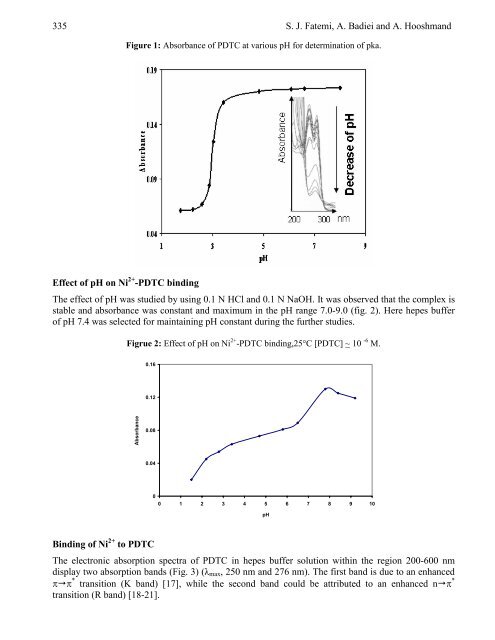
Figure 1: Absorbance <strong>of</strong> PDTC at various pH for determination <strong>of</strong> pka.<br />
Effect <strong>of</strong> pH on Ni 2+ -PDTC binding<br />
The effect <strong>of</strong> pH was studied by using 0.1 N HCl and 0.1 N NaOH. It was observed that the complex is<br />
stable and absorbance was constant and maximum in the pH range 7.0-9.0 (fig. 2). Here hepes buffer<br />
<strong>of</strong> pH 7.4 was selected for maintaining pH constant during the further studies.<br />
Figrue 2: Effect <strong>of</strong> pH on Ni 2+ -PDTC binding,25°C [PDTC] ~ 10 -6 M.<br />
Absorbance<br />
0.16<br />
0.12<br />
0.08<br />
0.04<br />
Binding <strong>of</strong> Ni 2+ to PDTC<br />
0<br />
0 1 2 3 4 5<br />
pH<br />
6 7 8 9 10<br />
The electronic absorption spectra <strong>of</strong> PDTC in hepes buffer solution within the region 200-600 nm<br />
display two absorption bands (Fig. 3) (λmax, 250 nm and 276 nm). The first band is due to an enhanced<br />
π�π * transition (K band) [17], while the second band could be attributed to an enhanced n�π *<br />
transition (R band) [18-21].