Physics for Geologists, Second edition
Physics for Geologists, Second edition
Physics for Geologists, Second edition
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
1 14 Fluids and fluid flow<br />
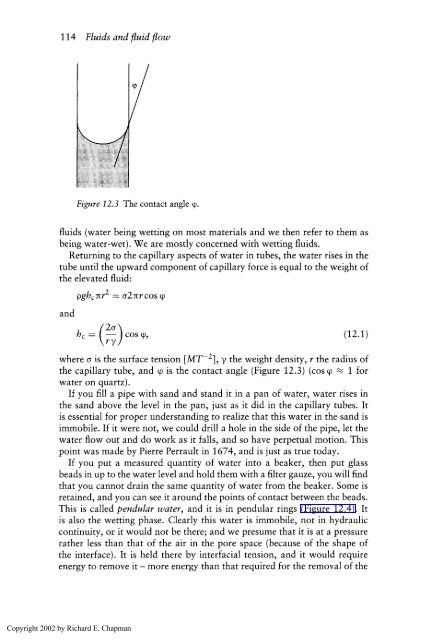
Figure 12.3 The contact angle 9.<br />
fluids (water being wetting on most materials and we then refer to them as<br />
being water-wet). We are mostly concerned with wetting fluids.<br />
Returning to the capillary aspects of water in tubes, the water rises in the<br />
tube until the upward component of capillary <strong>for</strong>ce is equal to the weight of<br />
the elevated fluid:<br />
and<br />
pgh,n? = o2nr cos cp<br />
h, = (g ) cos cp,<br />
where o is the surface tension [MT-2], y the weight density, r the radius of<br />
the capillary tube, and cp is the contact angle (Figure 12.3) (cos cp 1 <strong>for</strong><br />
water on quartz).<br />
If you fill a pipe with sand and stand it in a pan of water, water rises in<br />
the sand above the level in the pan, just as it did in the capillary tubes. It<br />
is essential <strong>for</strong> proper understanding to realize that this water in the sand is<br />
immobile. If it were not, we could drill a hole in the side of the pipe, let the<br />
water flow out and do work as it falls, and so have perpetual motion. This<br />
point was made by Pierre Perrault in 1674, and is just as true today.<br />
If you put a measured quantity of water into a beaker, then put glass<br />
beads in up to the water level and hold them with a filter gauze, you will find<br />
that you cannot drain the same quantity of water from the beaker. Some is<br />
retained, and you can see it around the points of contact between the beads.<br />
This is called pendular water, and it is in pendular rings (Figure 12.4). It<br />
is also the wetting phase. Clearly this water is immobile, not in hydraulic<br />
continuity, or it would not be there; and we presume that it is at a pressure<br />
rather less than that of the air in the pore space (because of the shape of<br />
the interface). It is held there by interfacial tension, and it would require<br />
energy to remove it - more energy than that required <strong>for</strong> the removal of the<br />
Copyright 2002 by Richard E. Chapman